Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Methotrexate (MTX) has historically been used as first-line therapy for juvenile localized scleroderma (jLS), but tolerability often limit compliance. Recent evidence from case series in LS and experience in treatment of skin disease in systemic sclerosis suggests that mycophenolate mofetil (MMF) may be equally effective in treating jLS with fewer side effects. The goal of this study compares treatment outcomes and tolerability between jLS patients treated with MTX and MMF.
Methods: Patients were selected from the National Registry of Childhood Onset Scleroderma (NRCOS). Inclusion criteria required a jLS diagnosis, being on MTX therapy, MMF therapy, or combination therapy (CT) of the two, and having at least two follow-up visits. Disease activity and damage were measured the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT). Side effects were self-reported. Statistical analyses included summary statistics, Chi-square test for side effect frequency, Wilcoxon signed-rank test for changes within groups, and Kaplan-Meier analysis with log-rank tests for disease flare incidence.
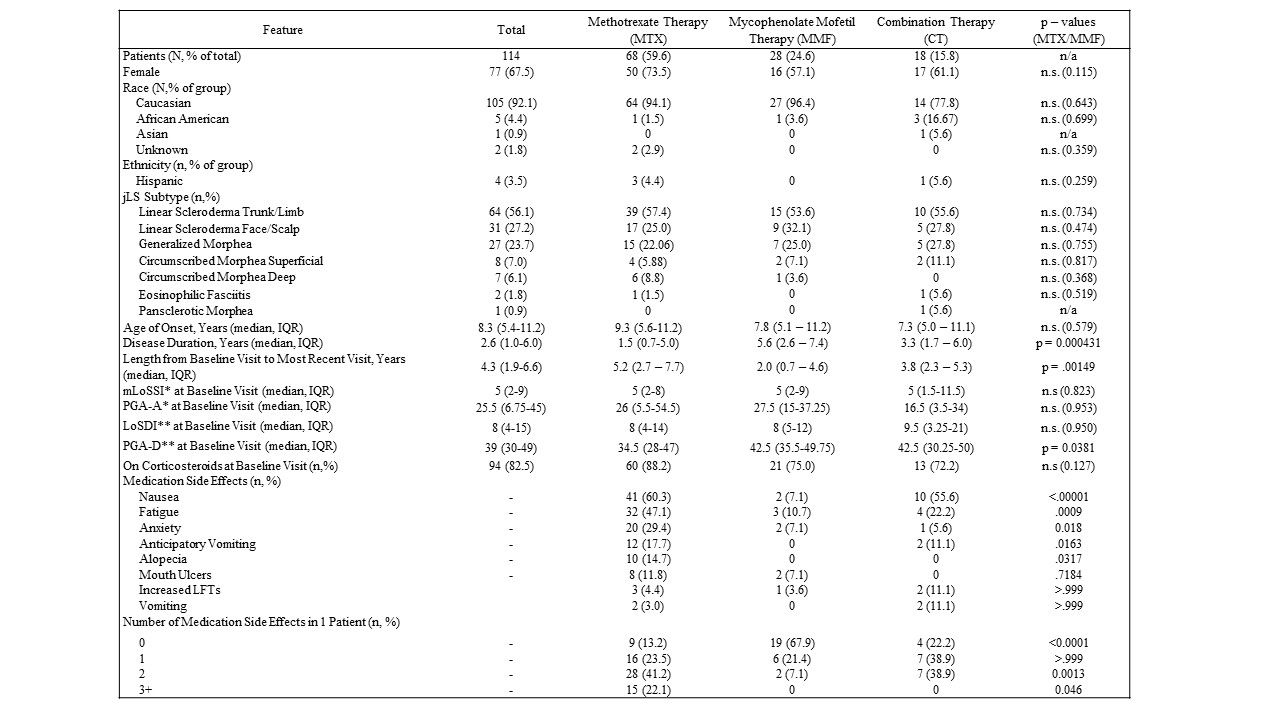
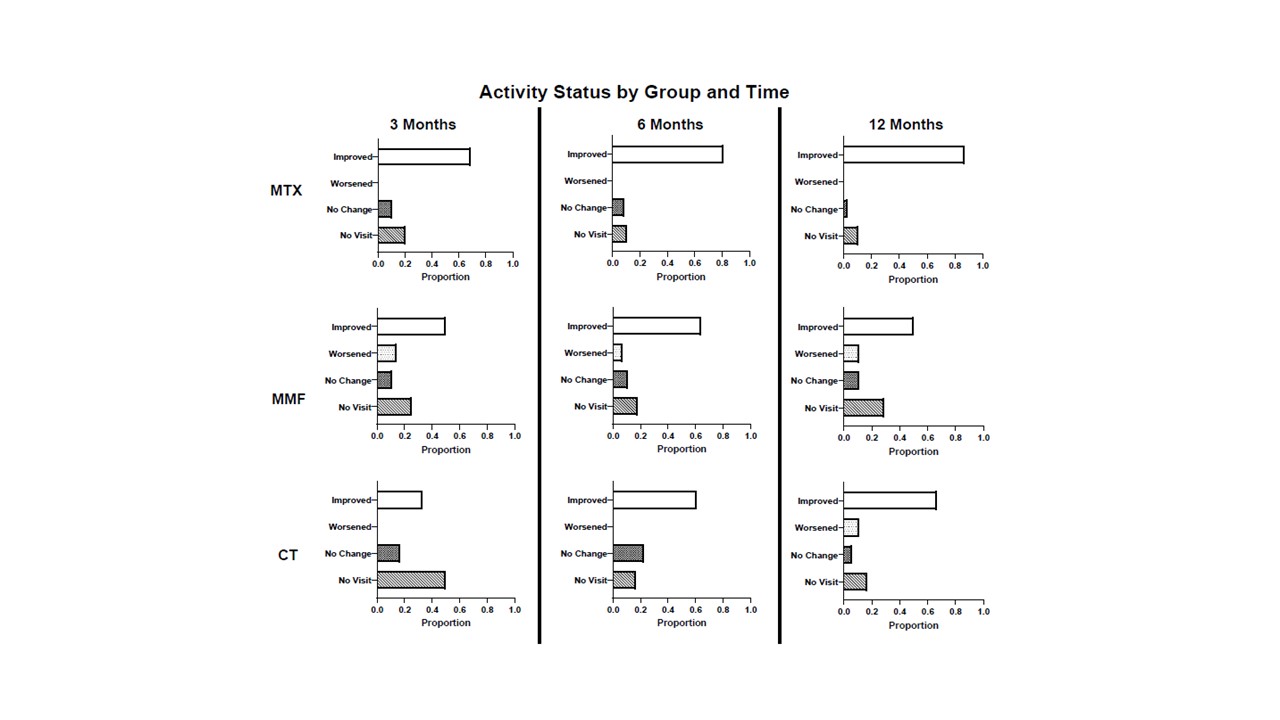
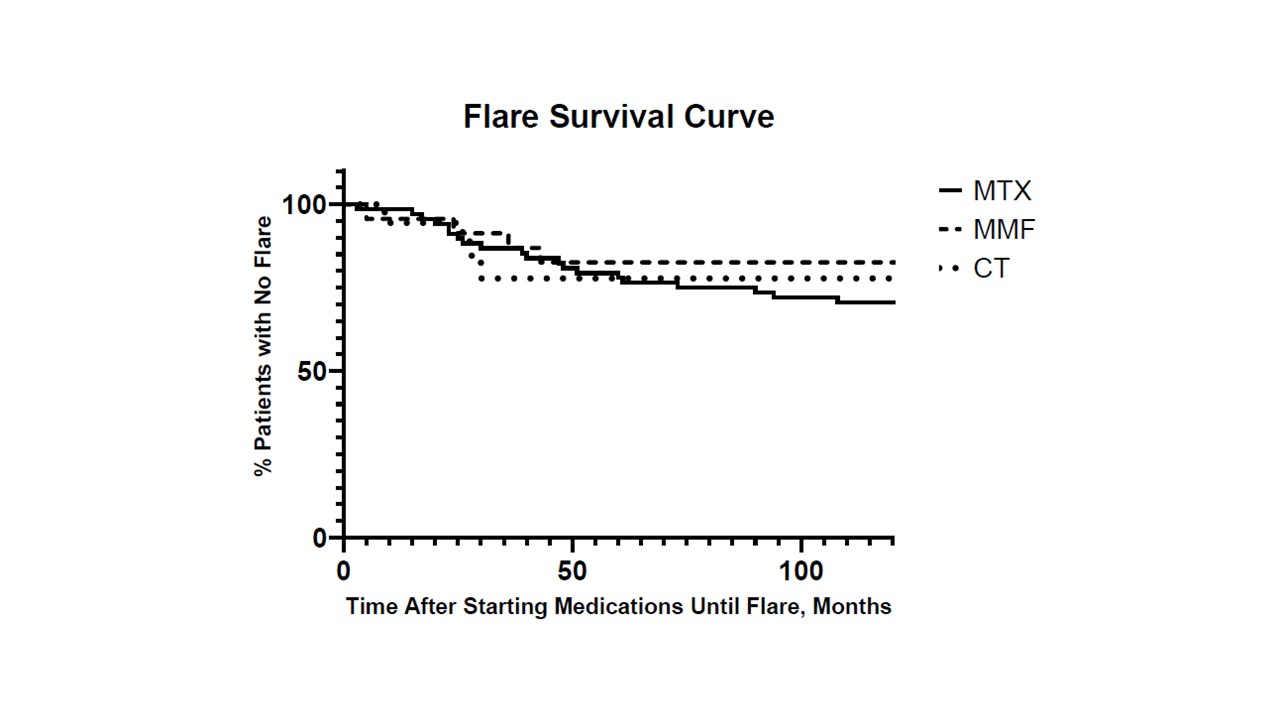
Results: The MTX, MMF, and CT groups included 68, 28, and 18 jLS patients, respectively, with no significant difference in baseline demographic information or disease subtype between groups (Table 1). MTX patients had a significantly shorter disease duration that MMF patients (1.5 vs. 5.6 years, p = 0.0004). Both MTX and MMF groups showed significant median disease activity decrease from baseline at three months (80%; p < 0.0001 and 100%; p = 0.012, respectively), and this decrease in scores was sustained at three, six, and 12 months, in both MTX and MMF groups (Figure 1). Median damage scores due to jLS remined stable. There was no significant difference in disease relapse rate over the follow-up interval (median 51.5 months) using Kaplan-Meier analysis (Figure 2). MTX patients reported significantly higher incidences of nausea (p < 0.0001), fatigue (p = 0.0009), and anxiety (p = 0.018) compared to MMF patients (Table 1).
Conclusion: Our analysis provides evidence that MMF is non-inferior to MTX in efficacy in an observational cohort study with no significant difference in both a) decrease in disease activity parameters at scheduled follow-up intervals and b) relapse rate of disease in jLS. Additionally, MMF patients experienced significantly fewer side effects. MMF monotherapy results also included patients who experienced disease flare during or after completion of MTX therapy regimens, confirming findings from previous literature about its usefulness as a second-line agent. While further controlled trials are needed, this provides evidence that MMF is equivalent to MTX and may be considered as a first line of treatment in jLS.
*Activity score subsections from the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT): mLoSSI = modified Localized Skin Severity Index, PGA-A = Physician Global Assessment – Activity
**Damage subsections from the LoSCAT: LoSDI = Localized Scleroderma Damage Index, PGA-D = Physician Global Assessment – Damage
To cite this abstract in AMA style:
DeRosas E, Havrilla H, Torok K. Evaluating the Non-Inferiority of Mycophenolate Mofetil Compared to Methotrexate in Treating Juvenile Localized Scleroderma [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evaluating-the-non-inferiority-of-mycophenolate-mofetil-compared-to-methotrexate-in-treating-juvenile-localized-scleroderma/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-the-non-inferiority-of-mycophenolate-mofetil-compared-to-methotrexate-in-treating-juvenile-localized-scleroderma/