Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Healthcare disparities in SLE clinical trials are well known, with minoritized individuals facing more significant SLE morbidity and mortality, while being underrepresented in randomized clinical trials (RCTs). However, we have limited information as to how to best recruit a diverse group of participants to SLE clinical trials. Barriers to clinical trial participation can originate both with the patients or with their clinicians. We therefore sought to investigate the effectiveness of educational curriculums at decreasing such barriers by improving the knowledge, skills and attitudes regarding clinical trial participation in both groups.
Methods: We collaborated with the American College of Rheumatology, the Lupus Research Alliance, and the Lupus Association to develop educational curriculums for both clinicians and patients to see if we could improve their knowledge, attitudes, and skills regarding clinical trial participation. Workshops lasted approximately 90 minutes for each group and had virtual and in-person options available. Participants were surveyed immediately before, immediately after and one month after the workshop to assess these domains. Data were analyzed using the Wilcoxson signed rank test and the T test for continuous variables and the McNemar’s test for categorical variables using SAS.
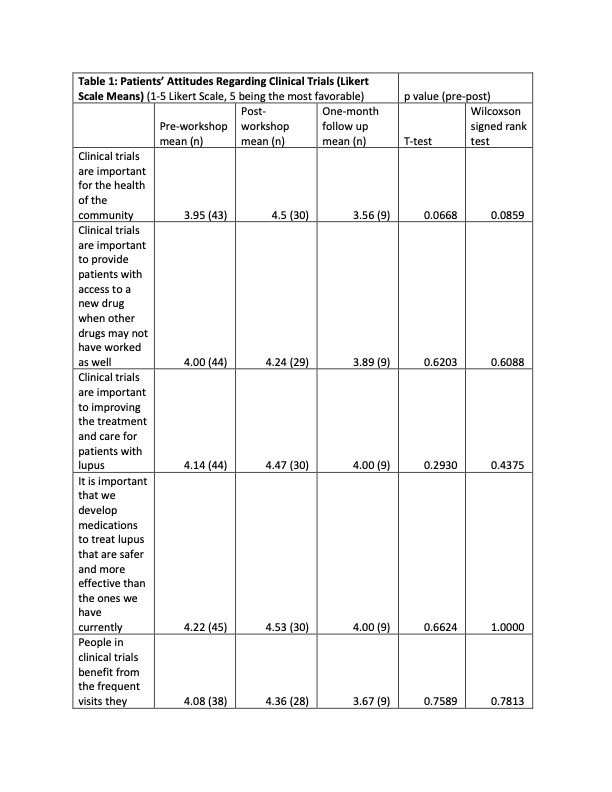
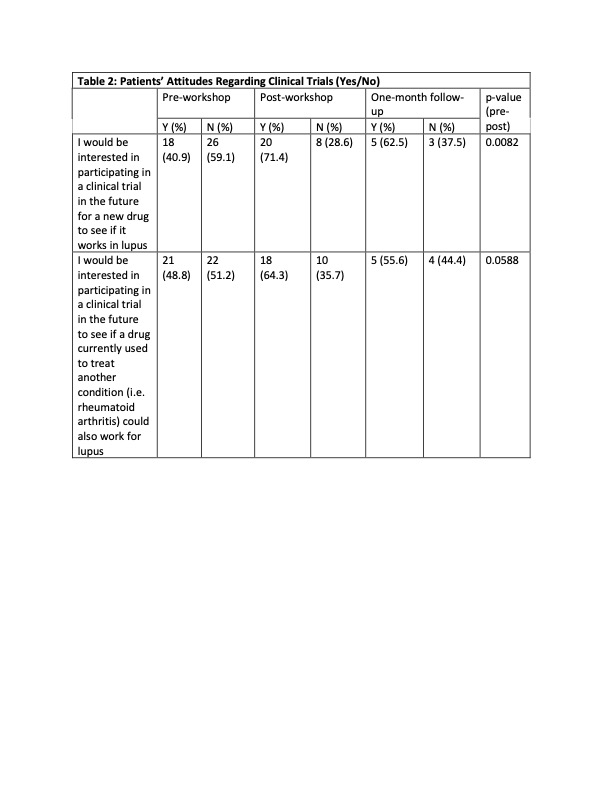
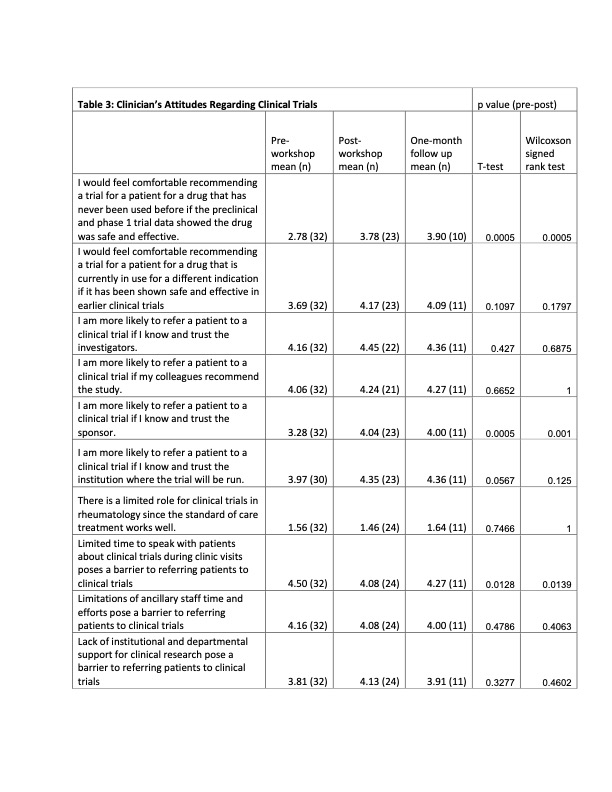
Results: 32 clinicians (physicians, fellows, advanced practice providers and RNs) participated in the clinician workshop, and 45 SLE patients participated in the patient workshop. Attitudes towards clinical trials were highly favorable among patients and clinicians prior to the workshop and remained positive afterwards. The workshops increased the patients’ comfort level regarding clinical trial participation and the protective benefits of the IRBs, and showed more interest for participating in a clinical trial. Clinicians reported comfort with the clinical trial process and how to refer patients to clinical trials. They also reported that they intended to discuss clinical trials more frequently with their patients. Regarding knowledge about clinical trials, the results were mixed. Clinicians reported an increased knowledge regarding protections afforded to patients in RCTs and SLE disparities, but knowledge about the historical and regulatory aspects of RCTs did not significantly improve. There was a significant increase in the patients’ knowledge regarding the protections afforded to them in clinical trials, but there was no significant change in their understanding of placebo and alternatives to clinical trial participation. However, these improvements did not translate into increasing patient enrollment into RCTs or research registries.
Conclusion: Although our study did increase both patients’ and clinicians’ skill and comfort level regarding RTC participation, its small size did not allow us to observe any concrete gains in RTC enrollment. This is a low-cost easily scalable solution, and larger studies done across different institutions could be done to conclusively answer whether educational initiatives have tangible benefits in patient recruitment.
To cite this abstract in AMA style:
Bacalao M, Maitre M, Kottur S. Evaluating the Effectiveness of Educational Initiatives on Recruiting Underrepresented Participants into SLE Clinical Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evaluating-the-effectiveness-of-educational-initiatives-on-recruiting-underrepresented-participants-into-sle-clinical-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-the-effectiveness-of-educational-initiatives-on-recruiting-underrepresented-participants-into-sle-clinical-trials/