Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The high costs of biologicals warrant a rational use of these drugs, preferably by tailoring them to the individual patient. The objective was to evaluate the cost-effectiveness of personalized biological treatment for rheumatoid arthritis (RA) using European League Against Rheumatism (EULAR) response and adalimumab drug level tests.
Methods: In 272 rheumatoid arthritis patients treated with adalimumab, Disease Activity Score in 28 joints (DAS28), Health Assessment Questionnaire (HAQ) and biological use was measured over three years. A treatment algorithm for personalized care was defined in which EULAR response and drug levels at 6 months defined whether adalimumab treatment was continued or discontinued, dosing was altered or, in case of non response, a next biological treatment was started.
Using a patient level Markov model, outcome in terms of DAS28 and HAQ and biological use according to the treatment algorithm for a personalized care group was simulated and compared to the observed drug use and disease course. Mean total costs, Quality Adjusted Life Years (QALYs) (both based on DAS28 and HAQ) and the average Incremental Cost-Effectiveness ratio (ICER) with 95 percentile range were calculated.
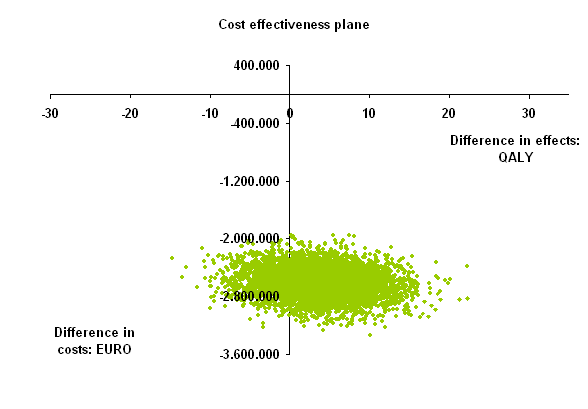
Results: Effectiveness was higher in the simulated personalized care group and the average difference in QALYs was 3.67 (95 percentile range -6.09 to 13.33). Costs were saved in the personalized care group as compared to observed care group: mean total savings €2.595.557 (95 percentile range -€2.983.760 to -€2.211755). In total €2.562.494 was saved on biological drug costs and testing costs amounted to €10.872. This resulted in an average ICER of -€ 707.236 per QALY gained. In 77.6% of simulations personalized care saved costs and was more effective (dominant) and in 22.4% cost-saving and less effective. Additionally, scenario analyses were performed and these were all cost-saving with variable effectiveness.
Conclusion: Tailoring biological treatment to individual RA patients using drug level tests to evaluate short-term outcomes is cost effective. Although specific for adalimumab, the results underline the potential cost-effectiveness for personalized biological treatment in RA.
|
|
observed care |
simulated care |
difference |
||
|
|
mean |
mean |
mean |
2.5% |
97.5% |
|
direct costs |
€4,263,470 |
€4,237,086 |
-€ 26,384 |
-€391,927 |
€334,886 |
|
productivity |
€1,876,147 |
€1,850,595 |
-€17,552 |
-€163,925 |
€124,873 |
|
drug costs |
€11,893,326 |
€9,330,832 |
-€2,562,494 |
|
|
|
testing costs |
€0 |
€10,872 |
€10,872 |
|
|
|
total costs |
€18,032,942 |
€15,437,385 |
-€2,596,557 |
-€2,983,760 |
-€2,211,755 |
|
QALYs |
587.98 |
591.65 |
3.67 |
-6.09 |
13.33 |
Disclosure:
C. L. M. Krieckaert,
None;
S. C. Nair,
None;
M. T. Nurmohamed,
MBS, MSD, Roche, Abbott, Pfizer and UCB,
5,
MBS, MSD, Roche, Abbott, Pfizer and UCB,
8;
C. J. J. van Dongen,
None;
W. F. Lems,
None;
F. P. J. G. Lafeber,
None;
J. W. J. Bijlsma,
None;
G. Wolbink,
Pfizer Inc,
2,
Pfizer Inc,
8,
Amgen,
8;
P. M. J. Welsing,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-the-cost-effectiveness-of-personalized-treatment-with-adalimumab-using-serum-drug-levels-in-rheumatoid-arthritis-patients/