Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Composite measures using 3-visual analog scale (VAS; physician’s global assessment, patient’s global assessment, and skin) or 4-VAS (physician’s global assessment, joints, skin, and pain) have been proposed as simpler alternatives.1 Given potential advantages of numeric rating scales (NRS) over VAS, we here adapted 3/4-VAS for use with NRS components and tested its validity via post hoc analysis of the upadacitinib (UPA) SELECT-PsA program.
We evaluate the ability of 3/4-NRS scores to assess treatment response in SELECT-PsA 1 and 2, as well as the correlation of 3/4-NRS with other common disease activity measures.
Methods: Data are from the SELECT-PsA 1 and 2 phase 3 trials in patients with prior inadequate response or intolerance to ≥1 non-biologic DMARD or ≥1 biologic DMARD, respectively. In both trials, patients received once daily UPA 15 mg, UPA 30 mg, or placebo (PBO); SELECT-PsA 1 also included the active comparator adalimumab (ADA) 40 mg every other week (wk). 3-NRS scores were determined using the mean of SAPS questions 1–10, physician’s global assessment of disease activity, and patient’s global assessment of disease activity; 4-NRS scores were determined using the mean of SAPS questions 1–10, physician’s global assessment of disease activity, patient’s assessment of pain, and BASDAI question 3 related to joint pain and swelling. The 3/4-NRS scale ranges from 0 (no disease activity) to 10 (severe activity). 3/4-NRS and cDAPSA (DAPSA without the CRP component) were assessed at all available visits through wk 56. Correlations between 3/4-NRS with PsA disease activity score (PASDAS), routine assessment of patient index data 3 (RAPID3), DAPSA, cDAPSA, and other disease activity measures were determined by nonparametric Spearman rank correlation coefficient for UPA 15 mg patients from both trials and ADA for SELECT-PsA 1. All data are shown as observed; nominal p-values are provided throughout.
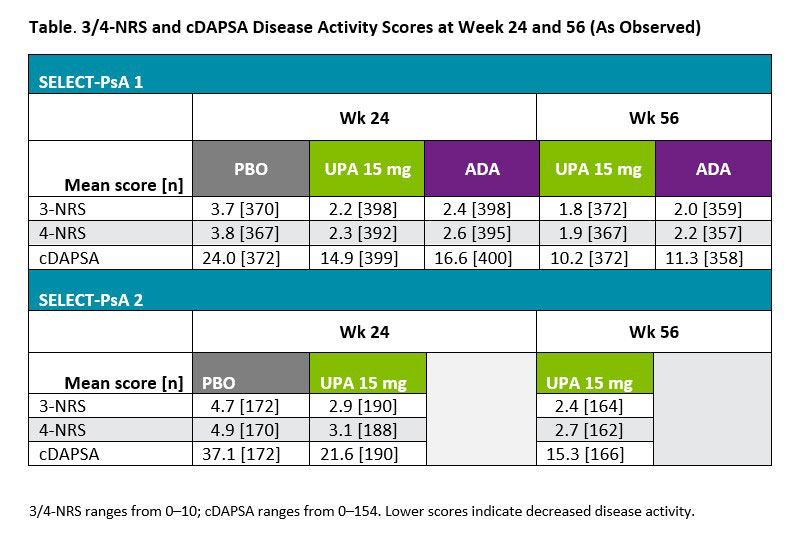
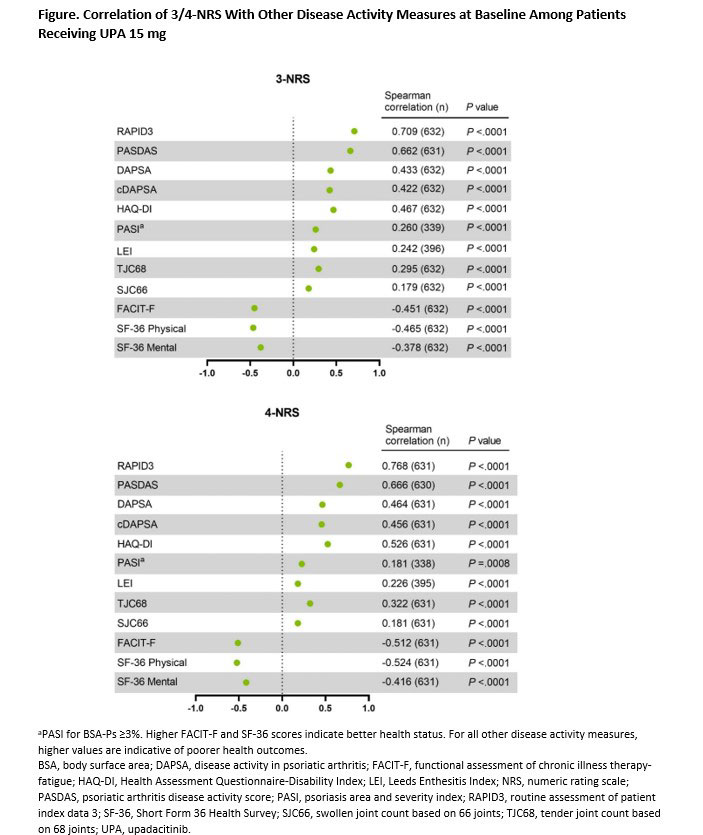
Results: A total of 1281 and 423 patients were included from SELECT-PsA 1 and 2, respectively. For both cDAPSA and 3/4-NRS scores, patients receiving UPA 15 mg showed clear numerical improvements compared with PBO at wk 24 in both trials (Table). 3/4-NRS scores were highly correlated with RAPID3 and PASDAS measures (r >0.6, P < 0.0001) for UPA 15 mg patients at baseline (Figure). Moderate correlations were observed between 3/4-NRS and DAPSA/cDAPSA (r = ~0.4, P < 0.0001), as well as physical function (HAQ-DI) and quality of life measures (SF-36). Nominally significant but weaker correlations were detected for joints, skin, and other disease activity assessments. Similar overall results were observed for patients receiving ADA.
Conclusion: 3/4-NRS was able to successfully discriminate between PBO and therapeutic groups in SELECT-PsA 1 and 2. 3/4-NRS scores correlated well with other clinical and patient reported outcome measures, including those focused on joints (DAPSA) or multiple manifestations (PASDAS), supporting 3/4-NRS as a viable and easy to use tool in daily clinical practice.
References
1. Tillett W, et al. J Rheumatol 2021; 201675.
To cite this abstract in AMA style:
Tillett W, Coates L, Kishimoto M, Setty A, Gao T, Lippe R, Helliwell P. Evaluating Numeric Rating Scale Versions of the 3 and 4 Visual Analog Scale (3/4-VAS) Composite Measures in Patients with Active Psoriatic Arthritis from the SELECT-PsA Program [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/evaluating-numeric-rating-scale-versions-of-the-3-and-4-visual-analog-scale-3-4-vas-composite-measures-in-patients-with-active-psoriatic-arthritis-from-the-select-psa-program/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-numeric-rating-scale-versions-of-the-3-and-4-visual-analog-scale-3-4-vas-composite-measures-in-patients-with-active-psoriatic-arthritis-from-the-select-psa-program/