Background/Purpose: We have earlier demonstrated that EuroQoL-5-Dimensions (EQ-5D) utility improves rapidly after commencement of tumor necrosis factor inhibition (TNFi) in rheumatoid arthritis (RA) and other arthritides, and that it is fairly stable for up to 7 years in those remaining on therapy(1). The development of utility over time in RA treated with other biologics is not well known.
Methods: Demographics, core set data, EQ-5D and data on drug treatment for patients with established RA on biologics from southern Sweden were retrieved from an observational database. Diagnosis was as by the treating rheumatologist, and has been shown to comply with 1987 ACR criteria in >95% of cases. Time frame was Jan 2006 – March 2014. EQ-5D utilities based on the British weights were computed and means and plotted over time.
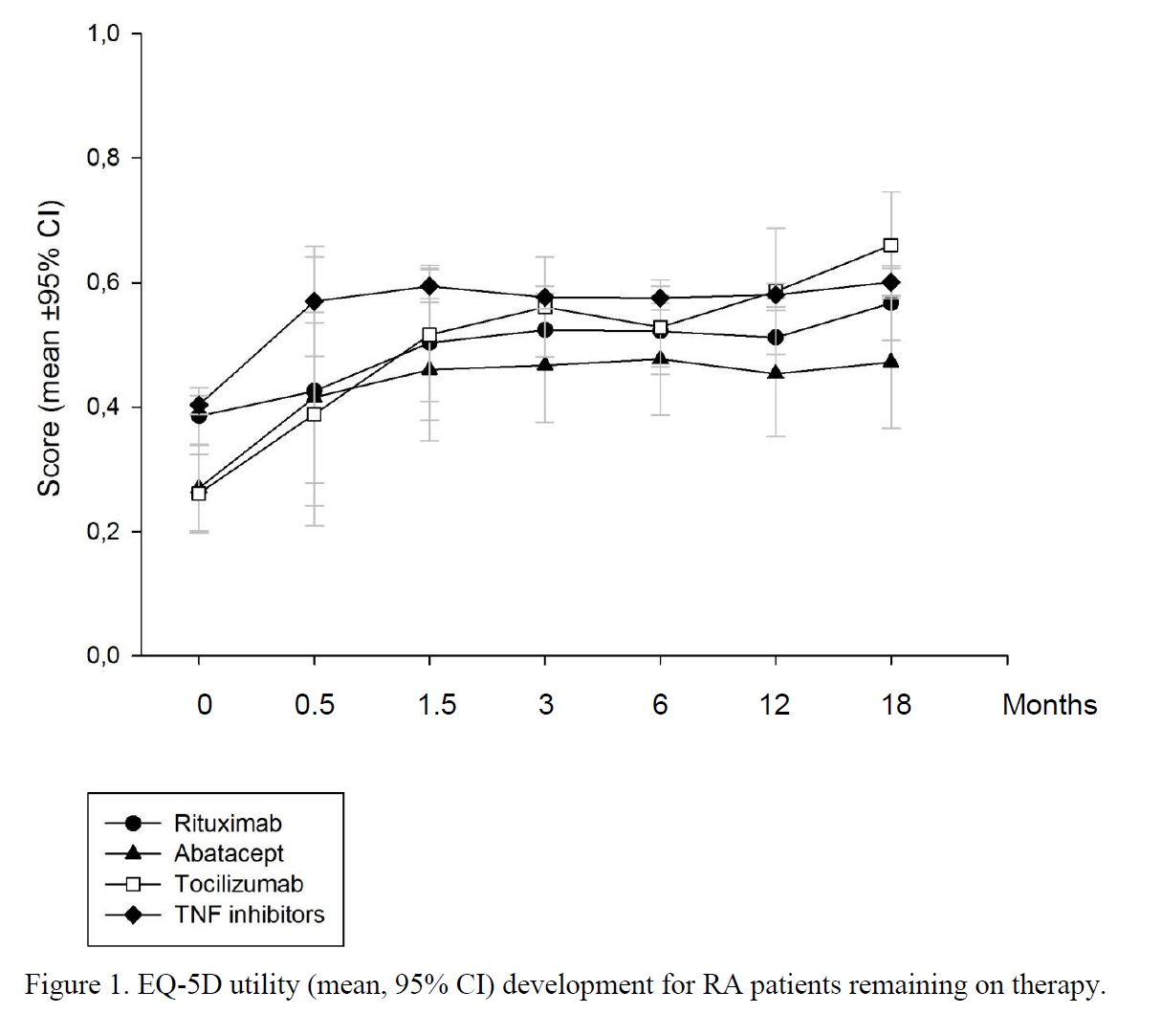
Results: There were 2418 patients treated with Abatacept (ABA), Rituximab (RTX), Tocilizumab (TOZ) or tumor necrosis factor inhibitors (TNFi) with utilities at treatment start (Table 1). Patients lacking baseline EQ-5D (n=913) did not differ appreciably from the main cohort (data not shown). TNFi patients had shorter disease duration and fewer previous DMARDs than patients on ABA, RTX or TOC, as these drugs were seldom started in bio-naïve patients. EQ-5D utility development over time is shown in Figure 1.
Conclusion: Despite starting at very low mean utilities, patients receiving ABA and TOC display rapid utility gains similar to TNFi although at a lower level, reflecting more longstanding and treatment resistant disease. As compared to the other biologics, the utility gain after commencement of RTX therapy starts at a level similar to TNFi (perhaps more bio-naïve patients with recent malignancy or other contraindications to TNFi) but is more gradual. Such differences may influence the area under the curve and thus accumulation of quality-adjusted life years. The continuing improvement observed in all groups may partly reflect a selection of patients responding and thus adhering to therapy.
Reference:
1. Gülfe A, Kristensen LE, Saxne T, Jacobsson LT, Petersson IF, Geborek P. Ann Rheum Dis. 2010 Feb;69(2):352-7.
|
Abatacept |
Rituximab |
Tocilizumab |
TNFi |
|
|
n |
100 |
230 |
121 |
1967 |
|
Age, years
|
59.0(12.1) |
60.2(12.3) |
57.9(13.5) |
56.6(13.6) |
|
Female, n(%) |
82(80.4) |
166(72.2) |
98(80.3) |
1520(77.3) |
|
Disease duration |
15.9(8.6) |
15.5(11.4) |
18.6(11.7) |
12.2(11.7) |
|
Baseline HAQ |
1.46(0.61) |
1.35(0.67) |
1.43(0.64) |
1.18(0.64) |
|
Baseline DAS28 |
5.50(1.41) |
5.04(1.57) |
5.68(1.34) |
5.10(1.37) |
|
Number of previous DMARDs*
|
5.9(3.2) |
5.3(2.8) |
5.2(2.8) |
3.0(1.9) |
|
Number of ongoing DMARDs**
|
0.7(1.0) |
0.8(0.6) |
0.7(0.6) |
0.8(0.6) |
|
Steroids, yes/no, n(%)
|
66(64.7) |
156(67.8) |
82(67.2) |
1164(59.2) |
|
Table 1. Baseline characteristics by therapy. Values are mean(SD) unless stated otherwise.
|
||||
|
TNFi, tumor necrosis factor inhibitors; DMARD, disease modifying antirheumatic drug; HAQ. Health assessment questionnaire.
|
||||
|
*including biologics
|
||||
|
**excluding ongoing biologic
|
||||
Disclosure:
A. Gülfe,
None;
J. A. Karlsson,
None;
L. E. Kristensen,
Abbvie, Pfizer, UCB, BMS, Roche, MSD,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/euroqol-5-dimensions-utility-gain-in-rheumatoid-arthritis-treated-with-abatacept-rituximab-tocilizumab-or-tumor-necrosis-factor-inhibitors/