Session Information
Date: Tuesday, October 23, 2018
Title: Vasculitis Poster III: Immunosuppressive Therapy in Giant Cell Arteritis and Polymyalgia Rheumatica
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Giant cell arteritis (GCA) represents the most common form of primary systemic vasculitis, and is frequently associated with comorbidities related either to the disease itself or induced by its treatment. Systematically collected data on disease course, treatment and outcomes of GCA remain scarce.
This EULAR Task Force therefore established a core set of data items which can easily be collected by clinicians, in order to facilitate collaborative research into the course and outcomes of GCA.
Methods: A multidisciplinary EULAR task force group of 20 experts including rheumatologists, internists, epidemiologists and patient representatives was assembled. A compilation of items describing GCA status and disease course was compiled to be discussed by three breakout groups during a one-day meeting. The results were presented to all members of the task force and further discussed. Final consensus was achieved by means of several rounds of email discussions after the meeting.
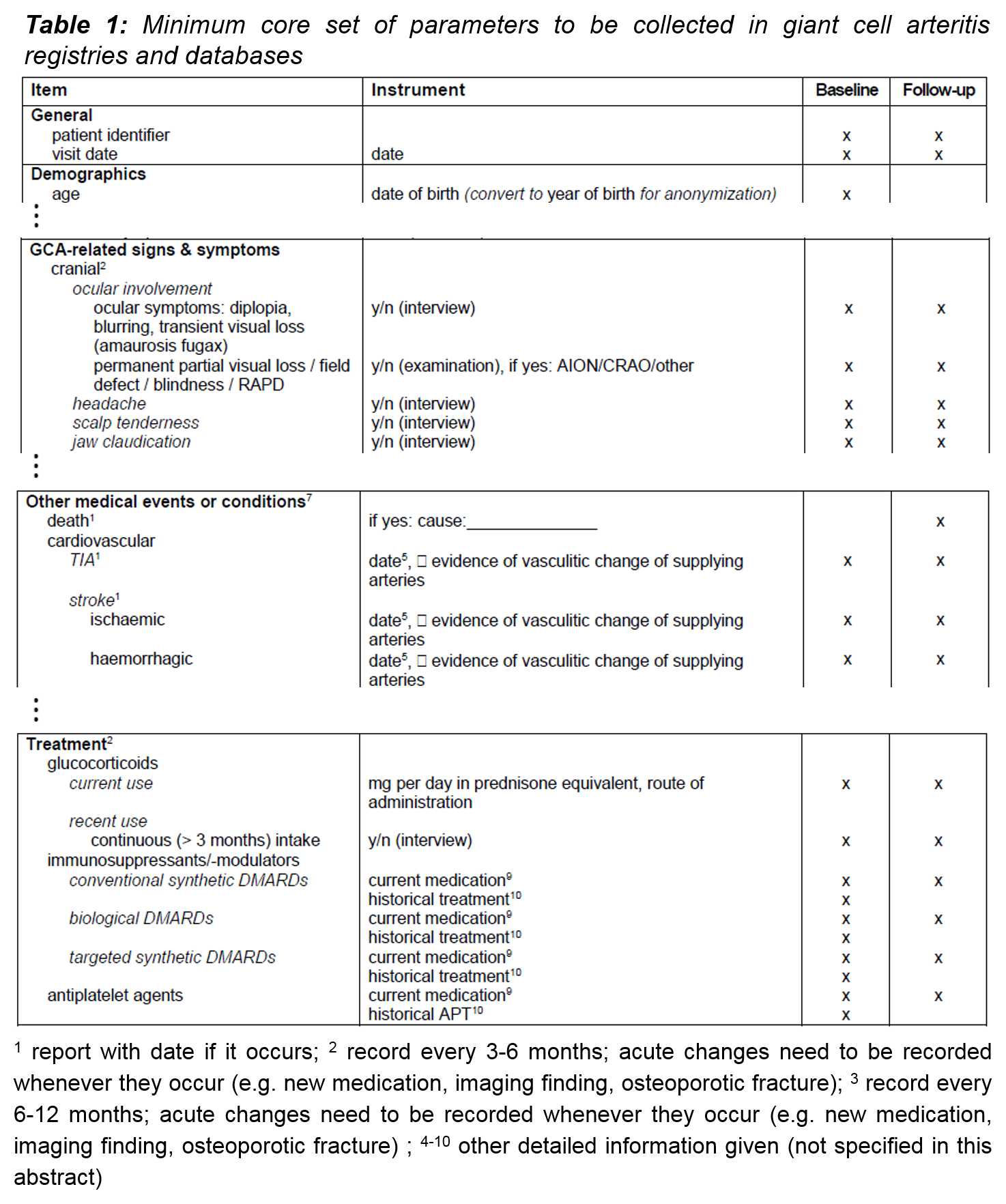
Results: Out of the original compilation, 95 parameters were considered relevant. Potential items were subdivided into the following categories: General, demographics, GCA-related signs and symptoms, other medical conditions, and treatment. Suitable instruments and assessment intervals were proposed for documentation of each item. In the next round of discussions the selection was reduced to a minimum core set of 70 parameters to facilitate implementation of the recommendations in both clinical care and clinical research. The following table represents an excerpt of the set of selected items. This is still a tentative listing since the final voting process on the choice of items is currently ongoing.
Conclusion: The recommended core set of parameters is intended to guarantee comparability of relevant items from different GCA registries and databases for the dual purposes of facilitating clinical research and improving clinical care.
To cite this abstract in AMA style:
Ehlers L, Askling J, Bijlsma JWJ, Cid MC, Cutolo M, Dasgupta B, Dejaco C, Dixon WG, Feltelius N, Finckh A, Gilbert K, Mackie S, Mahr A, Matteson EL, Neill L, Salvarani C, Schmidt WA, Strangfeld A, van Vollenhoven R, Buttgereit F. EULAR Task Force Recommendations for a Minimum Core Set of Parameters to be Collected in Giant Cell Arteritis Registries and Databases [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/eular-task-force-recommendations-for-a-minimum-core-set-of-parameters-to-be-collected-in-giant-cell-arteritis-registries-and-databases/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/eular-task-force-recommendations-for-a-minimum-core-set-of-parameters-to-be-collected-in-giant-cell-arteritis-registries-and-databases/