Session Information
Date: Monday, November 11, 2019
Title: Epidemiology & Public Health Poster II: Spondyloarthritis & Connective Tissue Disease
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In the expanding era of e-health, a wide range of mobile health applications (apps) have become available to enable people with rheumatic and musculoskeletal diseases (RMDs) to better self-manage their health. However, guidance on the development and evaluation of such apps is lacking. The objective of this EULAR task force was to establish points to consider (PtC) for the development, evaluation and implementation of apps for self-management of RMDs.
Methods: A systematic literature review of app content and development strategies was conducted, followed by a qualitative study with six patients and an online survey of people living with RMDs (n=394). Based on these data and expert opinion, the PtC were formulated in a face-to-face meeting in November 2018 by a multidisciplinary TF panel of experts, including patients, from 10 countries. The level of agreement among the panel in regard to each PtC was established by anonymous online voting.
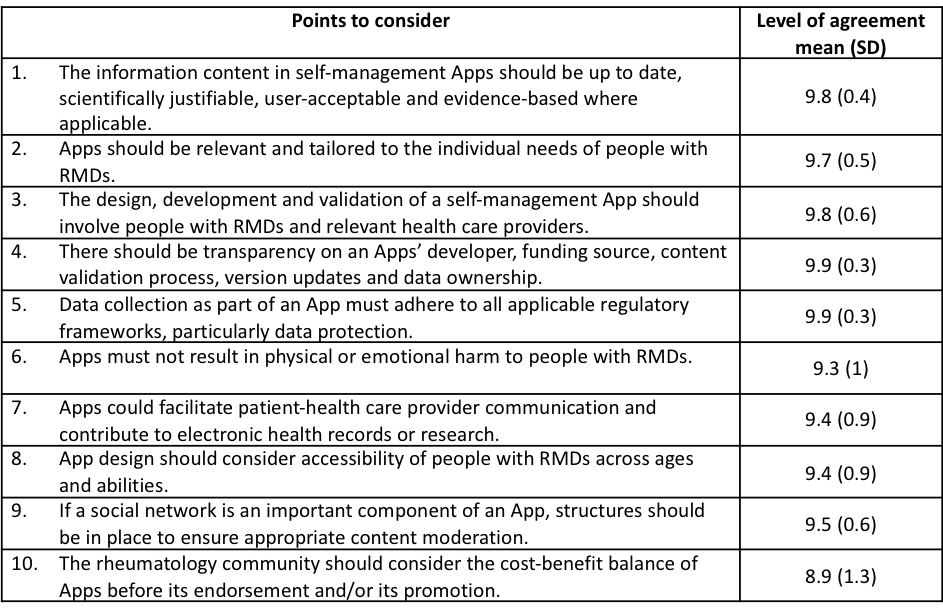
Results: Three overarching principles and 10 PtC were formulated (Table). Out of the 10 PtC, three were related to patient safety (1,5,6), considered as a critical issue by the panel, along with accuracy of information provided by apps. Three were related to relevance of the content and functionalities (2,7,9) and the importance of apps being tailored to the individual needs of people with RMDs. The requirement for transparency around app developers and funding sources (3,4), along with involvement of relevant health professionals were also raised. Ease of app access across ages and abilities was highlighted (8), in addition to considering the cost-benefit of apps from the outset (10). The level of agreement was high (Table).
Conclusion: These PtC provide guidance on important aspects that should be considered for the development of new apps, the quality assessment of existing apps, as well as for further development of existing apps.As part of the dissemination phase, these PtC will be shared with a larger group of health professionals, patients and app developers and for wider consensus.
To cite this abstract in AMA style:
Najm A, Nikiphorou E, Kostine M, Richez C, Pauling J, Finckh A, Ritschl V, Balazova P, Stones S, Szekanecz z, Prior Y, IAGNOCCO A, Ramiro S, Sivera F, Dougados M, Carmona L, Burmester G, Wiek D, Gossec L, Berenbaum F. EULAR Points to Consider for the Development, Evaluation and Implementation of Mobile Health Applications for Self-management in Patients with Rheumatic and Musculoskeletal Diseases [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/eular-points-to-consider-for-the-development-evaluation-and-implementation-of-mobile-health-applications-for-self-management-in-patients-with-rheumatic-and-musculoskeletal-diseases/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/eular-points-to-consider-for-the-development-evaluation-and-implementation-of-mobile-health-applications-for-self-management-in-patients-with-rheumatic-and-musculoskeletal-diseases/