Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Black, Indigenous, and people of color (BIPOC) experience greater morbidity and mortality from autoimmune connective tissue diseases (AICTDs); thus, equitable access to randomized controlled trials (RCTs) on AICTDs is a scientific and ethical imperative. Drug safety and efficacy may not be optimized until participants reflect the disease epidemiology. We aimed to examine race and ethnicity in RCTs investigating biologic and small molecule agents for AICTDs.
Methods: A search was executed on August 5th, 2022 across MEDLINE, Embase, Web of Science, Cochrane Library, Global Index Medicus, ClinicalTrials.gov, and International Clinical Trials Registry Platform for English-language studies involving biologics or new small molecule drugs for lupus erythematosus (LE), dermatomyositis, systemic sclerosis (SSc), or morphea. Three independent reviewers performed screening followed by eligibility assessment, with inclusion dependent on consensus by two reviewers. Duplicate study reports were removed, and data was summarized with descriptive statistics and chi-squared analyses.
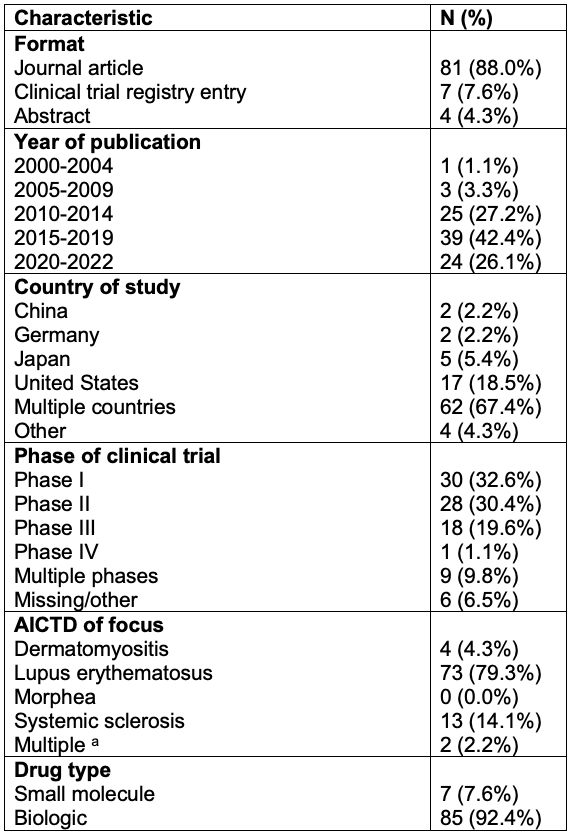
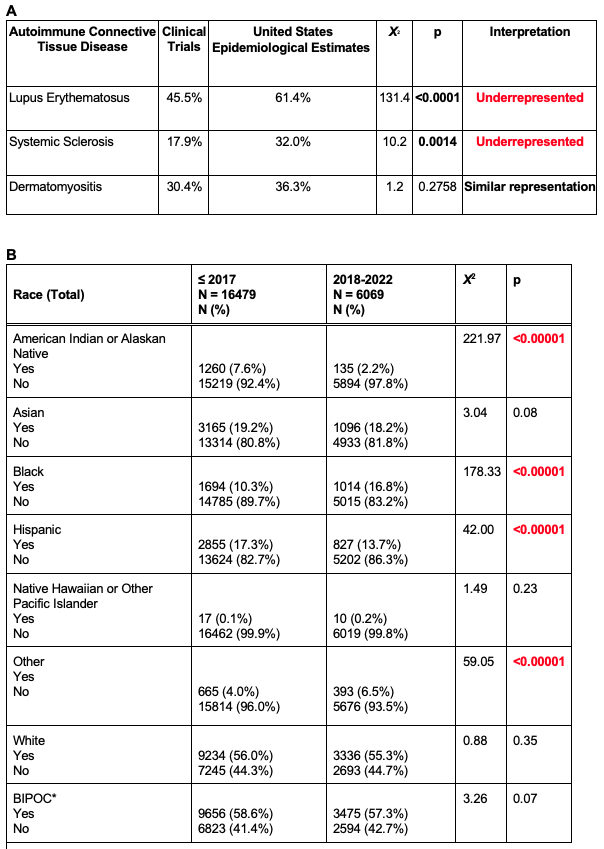
Results: Ninety-two RCTs were included. The most common reason for exclusion was a lack of participant race or ethnicity data; around three-fourths (74.5%) of otherwise eligible RCTs were excluded for this reason. Of the 92 included studies, 75 (81.5%) involved LE, 6 (6.5%) dermatomyositis, 14 (15.2%) SSc, and 0 (0.0%) morphea (Table 1). Across all AICTDs, when analyzing baseline demographics, patients who identified as White were the most frequently included (55.7%), followed by Asian (18.9%), Hispanic (16.3%), and Black/African American (12.0%). Those who identified as American Indian/Alaskan Native and Native Hawaiian/Other Pacific Islander were the least represented (6.2% and 0.1%, respectively). Chi-squared analysis revealed significant underrepresentation of patients who identify as BIPOC in United States (US)-based LE and SSc trials compared to United States epidemiological estimates (p< 0.0001 and p=0.0014, respectively; Fig 1, Table 2A). BIPOC were also underrepresented in US-based DM trials, but not significantly (p = 0.2758; Fig 1, Table 2A). World-wide, despite efforts for improved diversity in clinical trials, the inclusion of BIPOC in RCTs on AITCDs has not changed significantly in the last five years (X2 = 3.26, p = 0.07; Table 2B). When examining by race, inclusion of patients who identify as American Indian/Alaskan Native and Hispanic decreased (p< 0.0001). However, patients who identify as Black/African American increased (p< 0.0001), likely in part attributable to the EMBRACE study, a belimumab RCT which enrolled participants with African ancestry.
Conclusion: Our data show that inclusivity in RCTs for AICTDs remains problematic. This comes despite the recent push for greater diversity in clinical trial participants, with the understanding that race is a social construct, and that many differences in treatment response are driven by social, political, and environmental factors rather than biological factors. The lack of diversity in trials serves to exacerbate disparities in outcomes for patients who identify as BIPOC and are diagnosed with AICTDs.
*p<0.05
†Prevalence of disease by race/ethnicity was based on previously reported meta-analyses of Centers for Disease Control and Prevention registries for LE and reported 2020 prevalence of SSc and DM/polymyositis as mapped onto corresponding census data.
a Data is from 13 United States-based clinical trials involving 1240 patients with lupus erythematosus.
b Data is from 3 United States clinical trials involving 79 patients with dermatomyositis or polymyositis. United States epidemiological data includes patients with dermatomyositis or polymyositis who are 65 years old or younger.
c Data is from 4 United States clinical trials involving 112 patients with systemic sclerosis. United States epidemiological data includes patients with systemic sclerosis who are 65 years old or younger.
Epidemiological data for American Indian, Alaskan Native, Native Hawaiian, and Other Pacific Islander communities were not always available. Further, those who identified as “Other” race were not represented in the above figure. RCTs composed of healthy volunteers were not included.
a One RCT addressed SSc, LE, and DM; another RCT addressed LE and DM.
*American Indian/Alaskan Native, Asian, Black, Hispanic, Native Hawaiian/Other Pacific Islander, Other
To cite this abstract in AMA style:
Buechler C, Wanberg L, Rasner C, Theis-Mahon N, Hackman D, Pearson D. Ethnic and Racial Minorities in Clinical Trials for Autoimmune Connective Tissue Disease Therapeutic Agents [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ethnic-and-racial-minorities-in-clinical-trials-for-autoimmune-connective-tissue-disease-therapeutic-agents/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ethnic-and-racial-minorities-in-clinical-trials-for-autoimmune-connective-tissue-disease-therapeutic-agents/