Session Information
Session Type: Abstract Submissions (ACR)
Etanercept 25 mg once weekly program for rheumatoid arthritis, psoriatic arthropathy and ankylosing spondylitis patients in sustained clinical and radiological remission
Background/Purpose: Etanercept 50mg/week (ETN50) has demonstrated efficacy in patients with rheumatoid arthritis (RA), psoriatic arthropathy (PA) and ankylosing spondylitis (AS). In certain patients in sustained clinical remission with ETN 50, a dose reduction to etanercept 25 mg/week (ETN25) could be done. This strategy of dose reduction could have advantages in terms of safety and costs. The aim of the study is determine the clinical and economic impact of the use of ETN25 in RA, PA and AS patients in sustained clinical remission.
Methods: Observational, retrospective cohort of patients in an off-label program receiving ETN25 for at least 6 months between January 2006 and June 2013. Inclusion Criteria: patients treated with ETN50 that achieve and maintain clinical remission (DAS28<2.6 or BASDAI<2) during 1 year and slow worsening of structural changes were selected to change their standard dose of ETN50 to ETN25. We collected age, sex, indication, duration (in years) of ETN25 during the study period. In these patients, we simulated the cost of treatment with etanercept as if they had received ETN50 during their ETN25 respective periods. Economic impact was assessed using Enbrel® Spanish official prices.
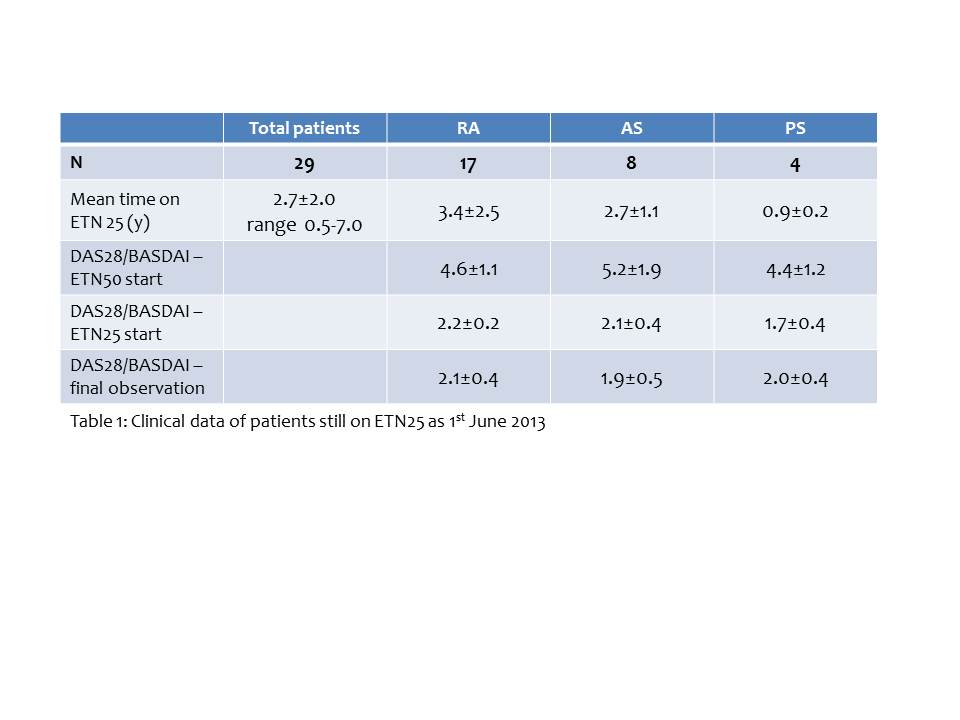
Results : From Jan 2006 to 1st Jun 2013, 39 patients (18 women; age 53±7 years; 24 RA, 7 PA, 8 AS) received ETN25 for at least 0.5 years (2.6±2.0 years; range 0.5-7.3 years). At 1st Jun 2013, 29 (74%) patients continued on ETN25 (17 RA, 4 PA and 8 AS). Table 1 shows associated clinical data of these patients. 10 (26%) patients discontinued due to: RA patients: 5 patients due to reactivation of RA (4 switched to ETN50 and 1 switched to adalimumab, all patients achieved clinical remission) and 2 patients due to adverse reactions; PA patients: 2 patients due to reactivation of PA (switched to ETN50 and achieved clinical remission) and 1 patient due to adverse reactions. All AS patients continued on ETN25 (Table 1). Total associated costs of this low dose strategy throughout the observation period were 622.073€. If these patients had been treated with ETN 50, the total cost of therapy would have been 1.224.146€. The implementation of the once-weekly ETN25 in these patients saved 622.073€ during 7 years. This cost savings achieved with an ETN25 regimen could lead to treat approximately 52 additional patients with RA, PA or AS for a year without increasing the total cost of etanercept therapy.
Conclusion: ETN25 produces important cost savings when used in patients with slow worsening of structural changes who maintain clinical remission for at least 1 year with ETN50. Reducing the dosage in selected patients could make treatment more cost-effective and allow physicians to treat more patients with a fixed budget.
Disclosure:
J. Borrás-Blasco,
None;
A. Gracia-Pérez,
None;
D. E. Casterá,
None;
J. D. Rosique-Robles,
None;
J. Abad,
None;
A. González Álvarez,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/etanercept-25-mg-once-weekly-program-for-rheumatoid-arthritis-psoriatic-arthropathy-and-ankylosing-spondylitis-patients-in-sustained-clinical-and-radiological-remission/