Session Information
Date: Monday, October 22, 2018
Title: 4M087 ACR Abstract: Reproductive Issues in Rheumatic Disorders (1852–1857)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Activation of the alternative and terminal attack complex of the complement system has been associated with adverse pregnancy outcomes (APOs) in stable/quiescent systemic lupus erythematosus (SLE). This prospective study examined erythrocyte bound C4d (EC4d), a marker demonstrating classical pathway activation, and complement C3/C4 levels in relationship to APO in pregnant SLE patients regardless of disease activity at entry.
Methods: Consecutive pregnant women (fulfilling ACR or SLICC criteria) were enrolled with no restrictions on disease activity or prednisone dosage. Serum C3 and C4 levels were measured using immunoturbidimetry and EC4d was measured from EDTA blood using flow cytometry and expressed as net mean fluorescence intensity (MFI). Whole blood Hydroxychloroquine (HCQ) levels were measured using liquid chromatography. Measures of disease activity consisted of Physician Global Assessment (PGA) visual analogue scale (0-3 cm) and clinical SLE Pregnancy Disease Activity Index (SLEPDAI) without immunology components. Estimates relating EC4d, complement C3 and C4 levels during gestation in the presence or the absence of APO events were analyzed using t-test and linear mixed effect models.
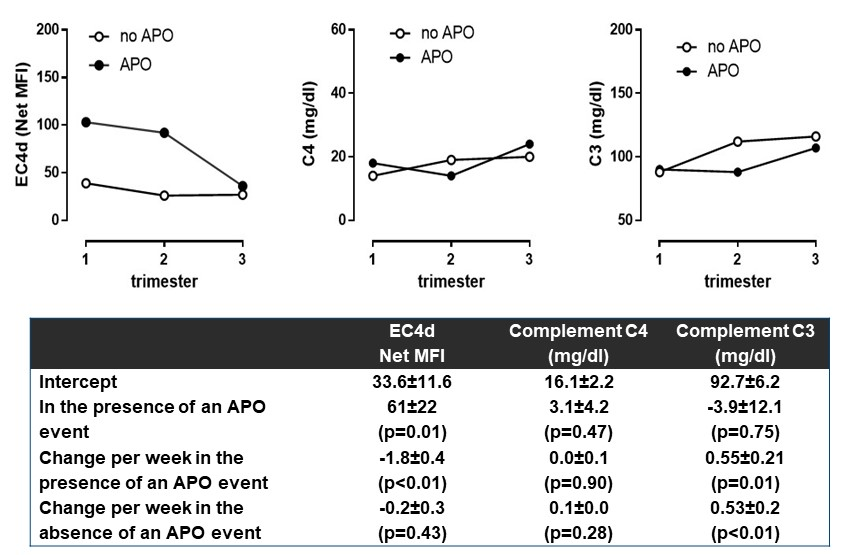
Results: A total of 29 consecutive women (mean age 32±1 years [SEM], 58% Caucasians and 21% African Americans) were enrolled and followed for an average of 3.1±0.2 visits during gestation. At baseline, mean PGA was 0.4±0.1 points and mean clinical SLEPDAI was 1.7±0.6 points, with 92% subjects ANA positive [titers≥1:80]. Six pregnancies (21%) resulted in APO events (two fetal deaths [at 27 and 28 weeks], three pre-term deliveries [range 27-35 weeks] and one delivery with small gestational age). The Figure highlights the change in EC4d, complement C4 and C3 levels in the patients with or without an APO. As presented in the Table and Figure, EC4d levels were higher during the first trimester in patients who went on to have APOs than in patients without APO (102±34 and 36±11 net MFI; p<0.05). In contrast, C3 and C4 levels were similar irrespective of APO status (p>0.47). During gestation, EC4d levels decreased by 1.8 units per week in those with APOs (p<0.01) but did not vary in those without APOs (estimate=-0.2; p=0.43). C3 increased during pregnancy irrespective of APOs (0.5 mg/dl per week). PGA and clinical SLEPDAI were not associated with APO (p>0.08). 25 patients were prescribed HCQ and there were no differences in the levels between the APO and non-APO groups (mean 1253±253 vs 850±196 ng/ml; p=0.28). In fact, all subjects with APO received HCQ and levels were >500 ng/ml (range 571-2139 ng/ml).
Conclusion: This prospective study suggests that EC4d is elevated early pregnancy in those destined for APOs, despite compliant HCQ levels in women with SLE. Classical pathway activation as measured by cell based complement products may be an important early biomarker and reveal pathogenesis of APO in SLE.
Disclosure: J. P. Buyon, Exagen, 2; P. M. Izmirly, Exagen, 2; H. M. Belmont, Exagen, 2; J. Conklin, Exagen Diagnostics Inc., 3; N. Kaiden, Exagen, 2; J. E. Salmon, None; R. Alexander, Exagen Diagnostics, Inc., 3; T. Dervieux, exagen, 3.
To cite this abstract in AMA style:
Buyon JP, Izmirly PM, Belmont HM, Conklin J, Kaiden N, Salmon JE, Alexander R, Dervieux T. Erythrocyte Bound C4d in the Presence of Adverse Pregnancy Outcome Events in Pregnant Women with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/erythrocyte-bound-c4d-in-the-presence-of-adverse-pregnancy-outcome-events-in-pregnant-women-with-systemic-lupus-erythematosus/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/erythrocyte-bound-c4d-in-the-presence-of-adverse-pregnancy-outcome-events-in-pregnant-women-with-systemic-lupus-erythematosus/