Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Hepatitis C virus (HCV) is associated with chronic wide spread pain, fatigue, myalgia, arthritis, sicca symptoms and vasculitis. Data regarding prior interferon-based treatment of HCV on pain is conflicting and the impact of direct acting antivirals (DAA) on extrahepatic manifestations is unknown. The study objective is to assess the impact of DAA treatment on pain and opioid use among patients treated at a single VA medical center.
Methods:
Data was obtained from the VA electronic health record through retrospective administrative data abstraction and manual chart review. Inclusion criteria: Veterans with positive HCV antibody or HCV RNA or HCV genotype test or an ICD 9/ICD 10 code for HCV, seen in the rheumatology clinic at least once during the study period and treated with DAA without interferon between January 1, 2010 and December 31st 2016; exclusion criteria: deceased during study period. In addition to demographics, HCV status and HCV treatment, data abstracted included (1) numeric rating scale pain scores (0=“no pain” to 10=”Worst pain imaginable”) obtained with other vital signs during outpatient visits and (2) opioid dose for patients prescribed opioid therapy. Pain scores were averaged over two 6-month periods: 6 months leading up to HCV treatment and 6 months following completion of treatment. Opioid dose was converted to a morphine equivalent daily dose (MEDD) and averaged across the same two 6-month intervals. Generalized estimating equations were used to model the change in average pain and MEDD from pre- to post-HCV treatment.
Results:
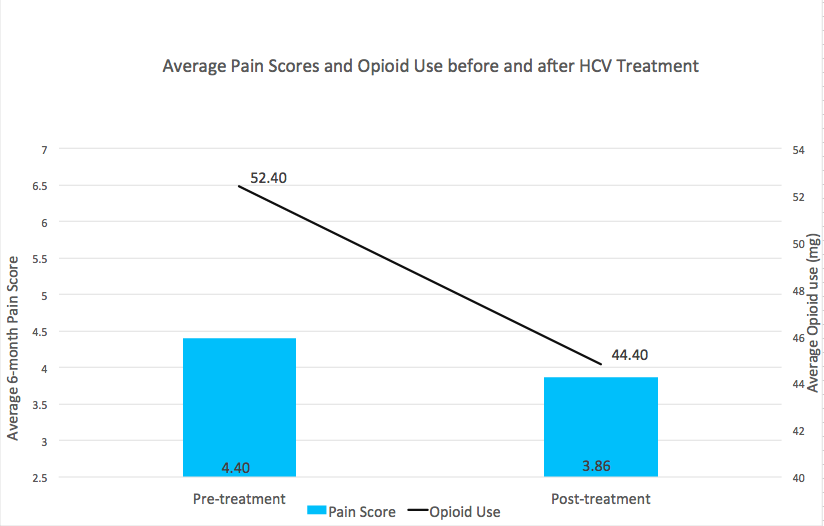
A total of 126 patients completed HCV treatment with DAA, of which 121 (96%) achieved a sustained virologic response (SVR) and were included in the analysis. A majority (93%) were male, with a mean age of 59 (± 5.6) years. Average pre-treatment pain was 4.4 (SD 2.4). Among the 67 patients prescribed opioid therapy, average pre-treatment MEDD was 52.40mg. Both pain and MEDD decreased following SVR. The effect of time was associated with an average reduction in pain of 0.54 points (p=0.02, Cohen’s d=0.16). Of the 67 patients prescribed opioids in the pre-treatment period, average MEDD decreased by 8mg during the post-treatment period (p<0.01, Cohen’s d=0.24), and 67% of patients experienced an opioid dose reduction, with 12 patients discontinuing opioids entirely.
Conclusion:
Among US Veterans with HCV seen in a rheumatology clinic at a single center, subjective pain scores were reduced post-treatment. In addition, among those prescribed opioids pre-treatment, a majority had a reduction in opioid dose post-treatment. Further evaluation of change in musculoskeletal manifestations of HCV and utilization of rheumatology care with the advent of highly effective HCV treatment is warranted.
Figure 1. Average pain score and opioid use before and after HCV treatment with direct acting antivirals. Opioid use is graphed as MEDD.
To cite this abstract in AMA style:
Kumthekar A, Shull S, Morasco B, Lovejoy T, Chang M, Barton J. Eradication of Hepatitis C Among US Veterans: Examination of Changes in Pain Severity and Prescription Opioid Use Following Treatment [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/eradication-of-hepatitis-c-among-us-veterans-examination-of-changes-in-pain-severity-and-prescription-opioid-use-following-treatment/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/eradication-of-hepatitis-c-among-us-veterans-examination-of-changes-in-pain-severity-and-prescription-opioid-use-following-treatment/