Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Knee osteoarthritis (KOA) is a leading cause of disability associated with risk of cardiovascular disease and reduced self-management of diabetes and hypertension. Recommended treatment includes intra-articular (IA) corticosteroids, however, these have limited duration of effect and risk of side effects. Patients with moderate KOA pain, not yet eligible for surgical intervention, may have unmet medical need as they are prescribed more pain medication yet report lower treatment satisfaction and decreased quality of life compared to patients with mild pain1. High BMI is a risk factor for KOA associated with increased knee pain potentially treatable with GLP-1 agonist therapy, however, patients with BMI < 30 who are not eligible for GLP-1 agonists present another potential unmet medical need.
EP-104IAR is a long-acting fluticasone propionate (FP) IA injection being developed for OA symptoms. EP-104IAR employs a novel controlled-release technology to optimize the pharmacokinetics of FP, maximizing IA residence time while limiting systemic exposure, providing a greater duration of efficacy with fewer systemic and local side effects.
Here we present the results of subgroup efficacy analyses of SPRINGBOARD, a Phase 2 randomized, double-blind, vehicle-controlled, parallel-group study (NCT04120402).
1 J Pain Res 2021; 14:2313-26
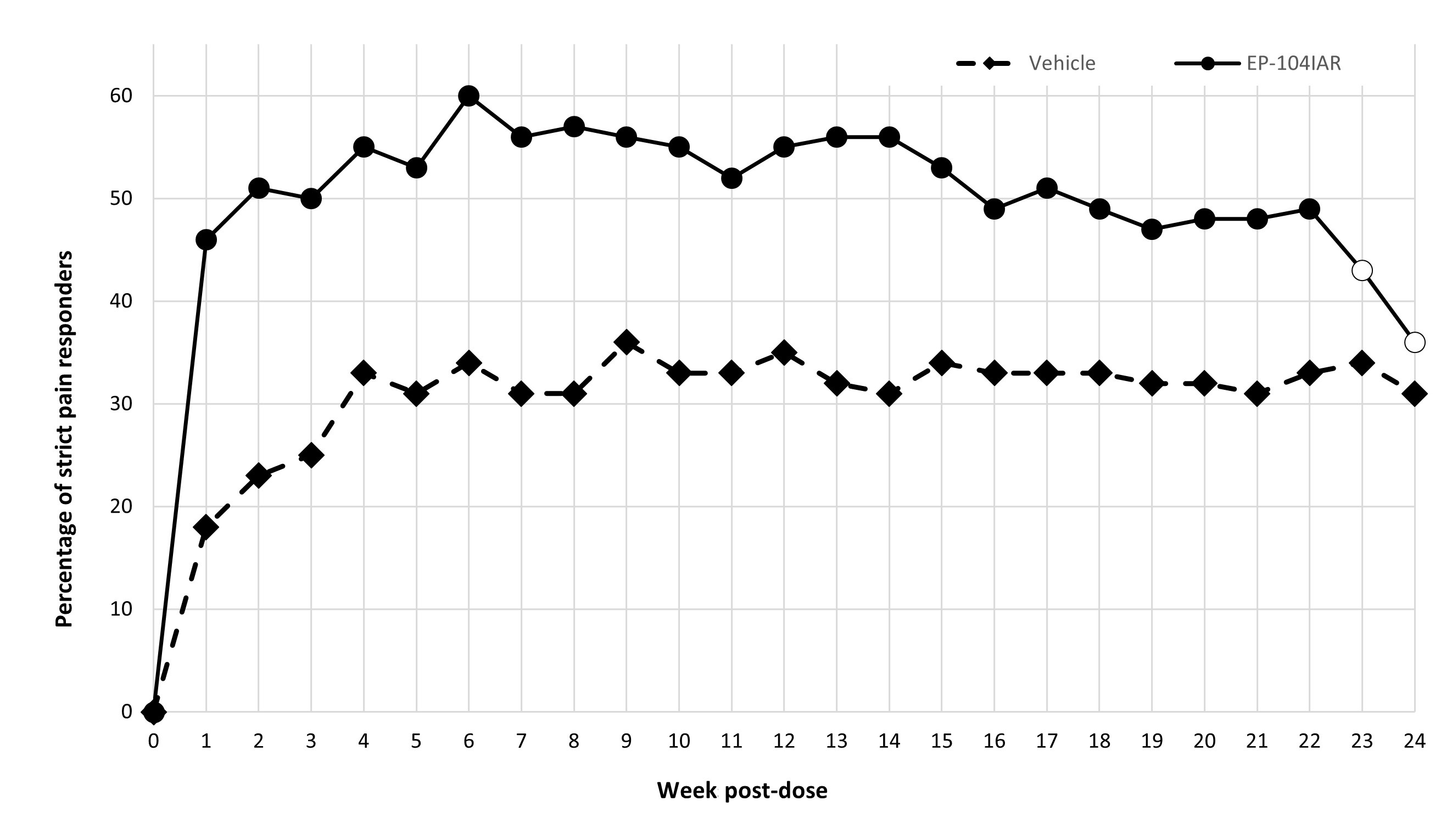
Methods: The study enrolled males and females, ≥ 40 years, with primary KOA with a Kellgren-Lawrence Grade 2 or 3, OA symptoms for ≥ 6 months and weekly WOMAC pain scores ≥ 4.0 to ≤ 9.0 (out of 10) which did not vary by > 3 points within the screening period. 318 subjects with KOA pain were randomized 1:1 to receive a single IA dose of EP 104IAR 25mg (163), or vehicle (155) in one index knee and were followed for 24 weeks. Of these, 214 (105 EP 104IAR, 109 vehicle) had moderate baseline WOMAC pain scores (3.5-6.5) and 168 (88 EP-104IAR, 80 vehicle) had BMI < 30. Weekly post-treatment WOMAC pain scores were collected and a mixed-effects model for repeated measures (MMRM) was fit to the change from baseline. The small amount of missing data was accounted for by the MMRM; no additional imputation was performed. Also, the proportion of pain responders, defined as ≥ 50% decrease from baseline with absolute decrease ≥ 2 was calculated for each week.
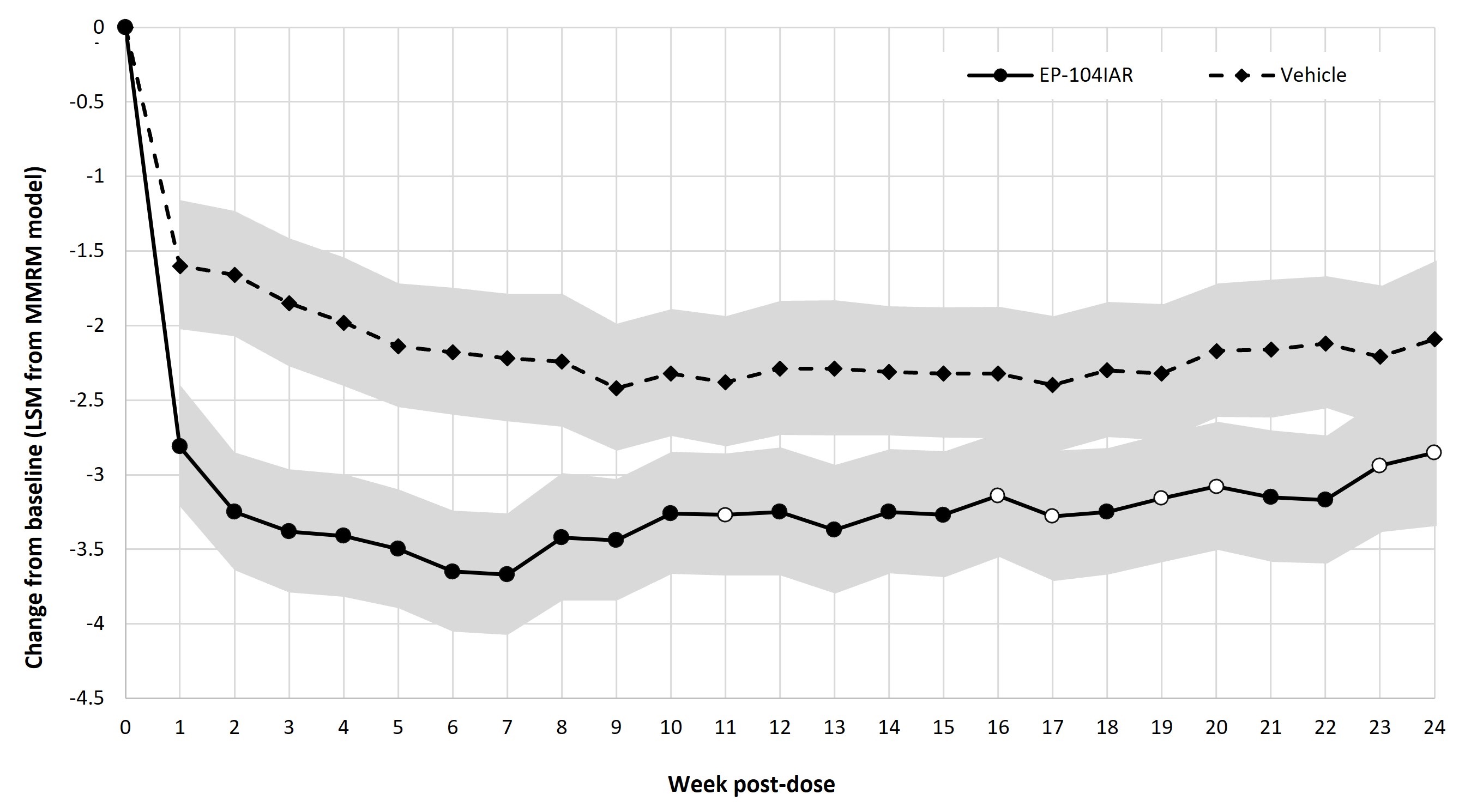
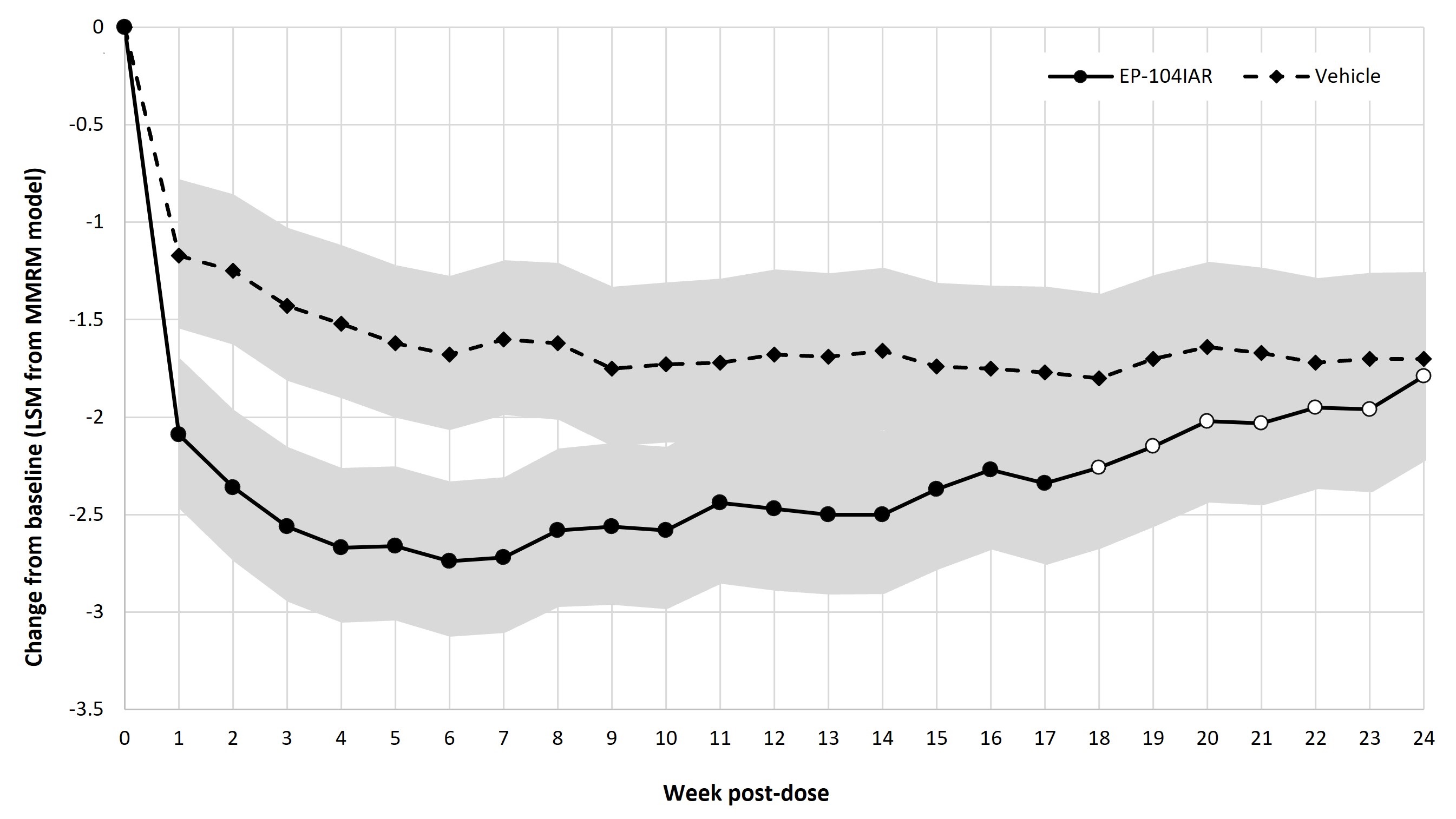
Results: Change from baseline in WOMAC pain at each week showed a statistically significant difference between EP-104IAR and vehicle at 17/24 timepoints in the BMI < 30 subgroup, including at Weeks 21 and 22 (Fig 1). A similar analysis in the moderate pain subgroup showed statistical significance to Week 17 (Fig 2). The frequency of pain responders was significantly greater in the EP-104IAR arm to Week 22 (Fig 3).
Conclusion: In this subgroup analysis of patients with moderate baseline pain and/or BMI < 30, a single dose of EP-104IAR provided statistically significant improvement in WOMAC scores for up to 22 weeks. This analysis reinforces that EP-104IAR has potential for sustained clinically meaningful benefit in KOA, which is of particular relevance in patients with moderate pain and/or reduced risk of progression to severe pain who may require treatment for a longer period of time before progressing to surgical intervention. Phase 3 trials of EP-104IAR are now planned.
Note: Solid dots indicate p < 0.05 in LS mean difference, open dots indicate p ≥ 0.05. Shaded areas indicate 95% CI
Note: Solid dots indicate p < 0.05 in LS mean difference, open dots indicate p ≥ 0.05. Shaded areas indicate 95% CI
Note: Pain response was defined as a decrease from baseline in WOMAC pain of at least 50% and 2 points
To cite this abstract in AMA style:
Malone A, Helliwell J, Kowalski M, Rovsing H, Lynggaard Boll S, Bihlet A, Prener Miller C, Mondragón A, Li Y, Dobek C, Peck V, Dye A, Wilmink M, Simon L, Conaghan P. EP-104IAR (Long-Acting Intra-Articular Injection of Fluticasone Propionate) Shows Sustained Improvement in Pain for Subjects with Moderate Baseline Pain and BMI Less Than 30 in SPRINGBOARD, a Phase 2, Randomized, 24-Week Study of Osteoarthritis of the Knee [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ep-104iar-long-acting-intra-articular-injection-of-fluticasone-propionate-shows-sustained-improvement-in-pain-for-subjects-with-moderate-baseline-pain-and-bmi-less-than-30-in-springboard-a-phase-2/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ep-104iar-long-acting-intra-articular-injection-of-fluticasone-propionate-shows-sustained-improvement-in-pain-for-subjects-with-moderate-baseline-pain-and-bmi-less-than-30-in-springboard-a-phase-2/