Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes III: Disease Activity
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: SLE patients suffer high symptom burden at the end-of-life. However, the course of disease and treatment burden in the last year of life have not been described. Also, there remains few predictors to identify patients at risk of imminent death within a year that could trigger initiation of supportive care.
Methods: We collected data from SLE patients (ACR/SLICC criteria) prospectively between 2013 to 2020 from 13 Asia-pacific countries. Data at each visit included laboratory variables, SLEDAI and treatment. SLICC Damage Index (SDI) and 36-item Short Form Survey (SF-36) were administered annually. We captured causes of death, with each death possibly attributed to more than one cause. We computed a modified SLICC-frailty index that excluded Sjogren syndrome, hypothyroidism, BMI, hypertension and headache disorder. In order to build a model to identify patients in need of supportive care at the end-of-life, we used a generalized estimating equations (GEE) model that included variables at a given visit that could predict imminent death within a year. Next, we used GEE models to compare the course of disease in the last year of life versus before, with respect to (i) flares; (ii) use of immunosuppressive agents (IS); (iii) corticosteroid dose (CS); (iv) treatment escalation or tapering (any increase or decrease respecitvely, in IS/CS doses); (v) visit intervals; (vi) SF36 physical (PCS) and mental (MCS) scores.
Results: We studied 4105 patients, of which 90 died at a median age of 43 (29.5-56.5) years and 7 (2-12) years after diagnosis. The most prevalent cause of death was infection (57/90, 63.3%) followed by SLE (40/90, 44.4%). The modified SLICC frailty index scores before death revealed that 73/90 (81.1%) were least fit or frail. In the last year of life, patients spent 56.9% of days on IS, the median CS dose was 10 (2.2-17.8) mg/day and visit interval was 85 (50-117) days; 41/90 (53.9%) had treatment escalated whereas 16/90 (21.1%) received treatment tapering.
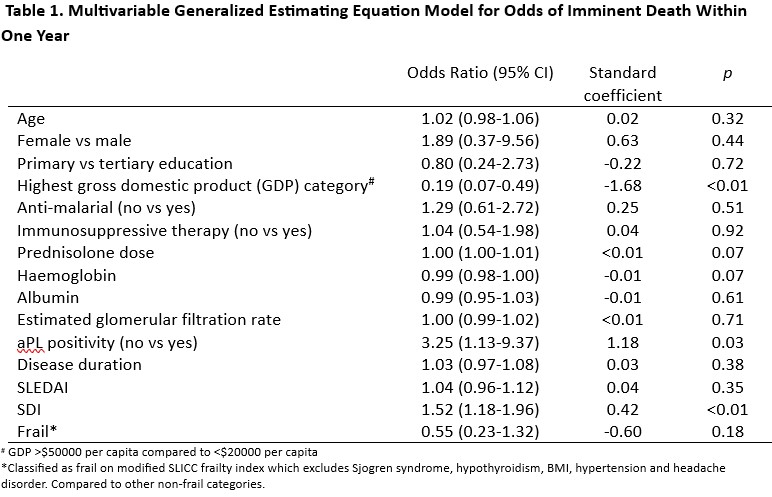
A model that included age, sex, anti-malarial use, country gross domestic product (GDP), haemoglobin, albumin, CS dose, SDI, SLEDAI and modified SLICC frailty was not sensitive (65.6%) or specific (73.4%) in identifying patients who would demise in a year. (Table 1).
In multivariable GEE models, the odds of flare were higher in the last year of life (OR 1.59, 95% CI 1.09-2.31, p=0.016). Patients had higher odds of staying in low disease activity (LLDAS) before the last year of life (OR 3.63, 95% CI 2.17-6.05, p< 0.01). Patients had fewer days on IS in the year preceding death, as compared to other years (p= 0.047), but had higher daily CS doses (p< 0.01) and shorter visit intervals (p< 0.01). Patients in the last year of life received more treatment escalations (OR 2.30, 95% CI 1.29-4.13, p=0.005). There were no significant differences in treatment tapering, PCS or MCS scores before or during the year preceding death.
Conclusion: In the year leading up to demise, SLE patients suffer increased flares and higher treatment burden. Existing disease-related instruments, laboratory and clinical variables have limited utility in identifying patients who would die within a year. Our results highlight an urgent need to better identify and support SLE patients near the end-of-life.
To cite this abstract in AMA style:
Cho J, Shen L, Kandane-Rathnayake R, Golder V, Louthrenoo W, Chen Y, Hamijoyo L, Luo S, Wu Y, Zamora L, Li Z, Sockalingam S, Katsumata Y, Harigai M, Hao Y, Zhang Z, Basnayake B, Chan M, Kikuchi J, Takeuchi T, Bae S, Oon S, O’Neill S, Goldblatt F, Ng K, Law A, Tugnet N, Kumar S, Tee C, Tee M, Ohkubo N, Tanaka Y, Navarra S, Lau C, Hoi A, Nikpour M, Morand E, Lateef A. End-of-Life in Systemic Lupus Erythematosus Beset by Increased Flares and Higher Treatment Burden: Data from a Prospective Large Multinational Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/end-of-life-in-systemic-lupus-erythematosus-beset-by-increased-flares-and-higher-treatment-burden-data-from-a-prospective-large-multinational-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/end-of-life-in-systemic-lupus-erythematosus-beset-by-increased-flares-and-higher-treatment-burden-data-from-a-prospective-large-multinational-cohort/