Session Information
Session Type: Poster Session A
Session Time: 6:00PM-7:00PM
Background/Purpose: Macrophage activation syndrome (MAS) is a life-threatening complication of different rheumatic diseases, particularly of systemic juvenile idiopathic arthritis (sJIA).
Methods: We report the case of 17-year-old girl with sJIA complicated by recurrent severe MAS episodes who received emapalumab (anti-IFNg antibody) in two subsequent MAS episodes and then underwent an uncomplicated hematopoietic stem cell transplantation (HSCT) while receiving emapalumab and anakinra granting complete control of inflammatory activity of the underlying disease.
Results: A 13-year-old White Caucasian girl presented with fever, rash and hepato-splenomegaly. Laboratory parameters were consistent with full-blown MAS (table 1). In the absence of clear evidence of an underlining condition, a diagnosis of secondary HLH was made, treatment with high dose of intravenous (IV) methylprednisolone (mPDN) and oral cyclosporine (CYC) was started with progressive improvement. After one year, still on CYC, she presented with fever, rash and arthritis with laboratory parameters consistent with MAS (table 1). Diagnosis of sJIA complicated by MAS was made. In 24 hours, she rapidly worsened and was admitted in ICU. High dose of IV mPDN (7 pulses of 30 mg/kg/day) as well as IV CYC (5 mg/kg/day) did not yield a response. Emapalumab was started, in the NI-0501-06 trial, (6 mg/kg initial dose followed by 3 mg/kg every 3 days) for 11 infusions. Conditions progressively improved. In order to prevent flares of the underlining sJIA, anakinra (2 mg/kg/day) was started. After 2 years in clinical remission, while she receiving anakinra every other day, she presented with fever, vomiting and diarrhea. Anakinra was immediately increased to daily dosing. Stool analysis showed Salmonella infection and antibiotic therapy was started. Nevertheless, she rapidly worsened, laboratory parameters were again consistent with full-blown MAS (table 1). She required ICU admission for multiorgan failure. Anakinra was administered IV and the dose increased up to 12 mg/kg/day. IV MPDs (8 pulses of 30 mg/kg/day) as well as IV CYC (5 mg/kg/day) were started with partial response. Based on her previous response to emapalumab, emapalumab was started again (compassionate use) with marked and rapid improvement. Because of recurrent MAS episodes and particularly for their rapidly evolution, the patient underwent an ex-vivo T cell-depleted haploidentical HSCT from her mother. The conditioning regimen was based on a Thiotepa-Treosulfan-Fludarabine scheme. Emapalumab was continued 1 month after HSCT together with anakinra. The patient achieved full donor engraftment with complete donor-derived immune reconstitution after 3 months. One year after HSCT, she is in excellent clinical condition on anakinra every other day, with complete remission of sJIA/MAS, also confirmed by persistently normal levels of IL-18 and CXCL9 (table 1).
Conclusion: This case provides further evidence of the efficacy of emapalumab in MAS, of the potential benefit of HSCT in difficult to treat sJIA patients. Notably, full control of inflammatory activity with emapalumab and anakinra may help to obtain a successful HSCT and reduce the risk of rejection.
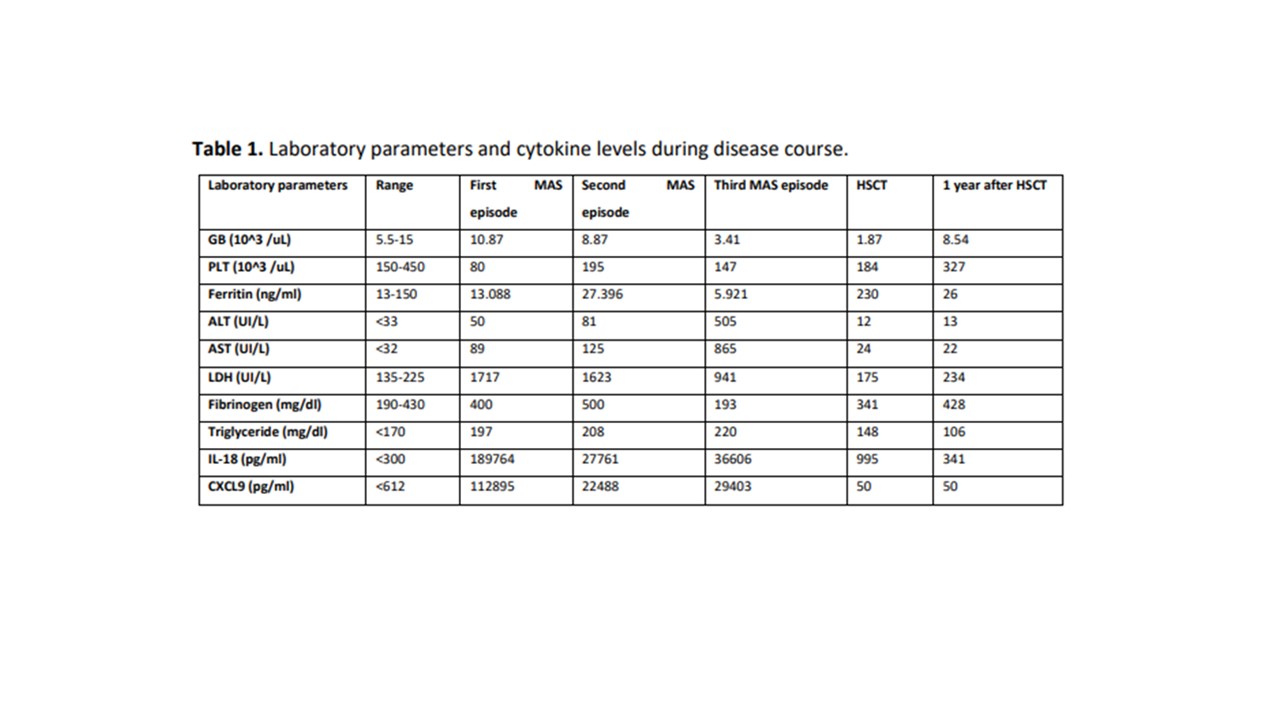 Table 1. Laboratory parameters and cytokine levels during disease course.
Table 1. Laboratory parameters and cytokine levels during disease course.
To cite this abstract in AMA style:
Bracaglia C, Pardeo M, Marucci G, Riccio S, Quagliarella F, Caiello I, Prencipe G, Merli P, Locatelli F, De Benedetti F. Emapalumab Treatment Followed by Hematopoietic Stem Cell Transplantation in Systemic Juvenile Idiopathic Arthritis Complicated by Recurrent Macrophage Activation Syndrome [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/emapalumab-treatment-followed-by-hematopoietic-stem-cell-transplantation-in-systemic-juvenile-idiopathic-arthritis-complicated-by-recurrent-macrophage-activation-syndrome/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/emapalumab-treatment-followed-by-hematopoietic-stem-cell-transplantation-in-systemic-juvenile-idiopathic-arthritis-complicated-by-recurrent-macrophage-activation-syndrome/
