Session Information
Date: Sunday, November 8, 2015
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment Poster Session I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Enhancing a typical EHR through implementation and utilization of research-validated instruments is termed Electronic Data Capture (EDC). EDC is found to be more efficient than standard paper-based data collection systems for accuracy, timeliness, speed and quality. We report our experience in establishing a validated lupus cohort via EDC for clinical, quality improvement and translational research and to determine predictors of damage index as the initial validation step
Methods:
Since January 2014 customized, lupus-specific documentation flow sheets such as the Systemic Lupus Erythematosus (SLE) disease activity index (SLEDAI) and Systemic Lupus International Collaborating Clinics (SLICC) damage index (SDI) were built and deployed within our Epic Care EHR for all patients with a 710.0, ICD-9 diagnosis of SLE when seen in the rheumatology clinic. Diagnosis was retrospectively validated by the SLICC classification criteria and patients met at least four. A retrospective-prospective cohort study design was implemented in which the SLE indices were linked to longitudinal EHR data collected every visit. The EHR data include demographics, vitals, comprehensive past history, medications, and labs. An example of data linking included the aligning of lab results, which shortly preceded or immediately followed clinic visits during which SLE indices scores were recorded. All statistical analyses were performed using SAS 9.4.
Results:
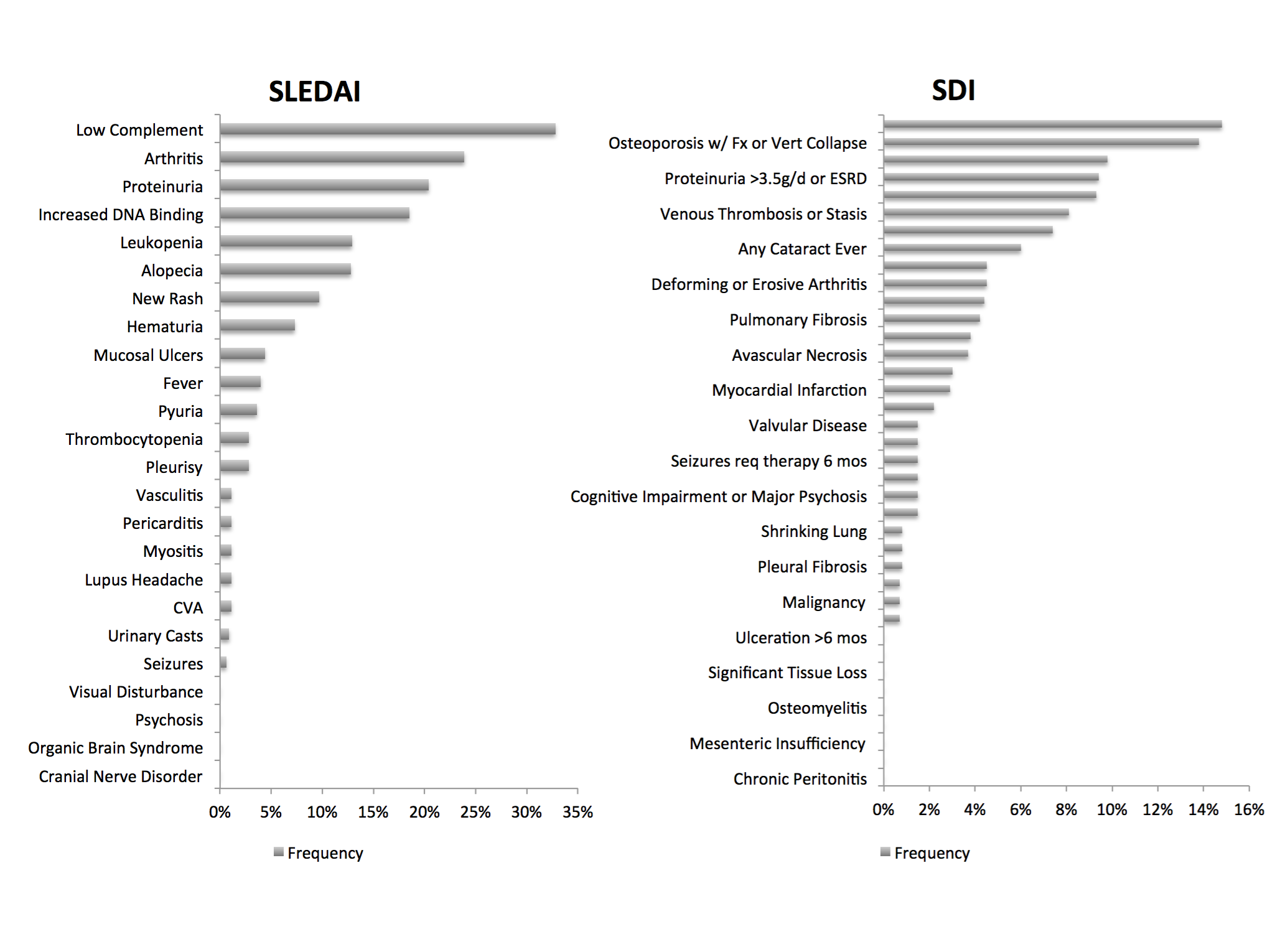
180 patients were enrolled in the cohort. 61.1% were African American and a significant number were hypertensive (60%). Mean SLEDAI was 3.6. Mean SDI (n=137) was 1.4. 62% had an SDI score of ³ 1. The frequency of SLEDAI and SDI components is shown in figure 1. Factors associated with worse SDI were older age (p=0.001), female gender (p=0.09), presence of hypertension (p=0.002), positive Smith/RNP (p=0.10) antibody, elevated ESR (p=0.006) and CRP (p=0.07), use of antiplatelet/anticoagulant agents (p=0.01). Hydroxychloroquine use (79.4%) (p=0.0005), positive SS-A/B (p=0.03/0.07) and pleuritis (p=0.03) were associated with lower damage. The association of worse damage with age, hypertension and protective effect of hydroxychloroquine were analogous to the SLICC and Hopkins lupus cohort.
Conclusion:
EHR-EDC is a powerful tool for clinical and translational research. The careful design, validation and integration within the EHR along with lupus indices allow regular automatic data extraction. Therefore with the increasing availability of EHR, the generation of cohorts for lupus and other diseases should also be achievable by centers with limited clinical research resources. Finally EHR-EDC also enables routine performance assessment for continuous improvement of care for lupus patients. Our lupus cohort had significant cardiovascular burden and was associated with worse damage, therefore requiring aggressive preventive measures.
Figure 1
To cite this abstract in AMA style:
Kothandaraman S, Ramsey F, Fleece D, Sorenson A, Ping L, Mukkera S, Goh K, Caricchio R. Electronic Health Record (EHR) As a Powerful Tool to Establish Clinical Research Lupus Cohorts [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/electronic-health-record-ehr-as-a-powerful-tool-to-establish-clinical-research-lupus-cohorts/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/electronic-health-record-ehr-as-a-powerful-tool-to-establish-clinical-research-lupus-cohorts/