Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The approach to elderly patients (≥60 years) with rheumatic diseases offers certain difficulties and uncertainties. Older adults are a particularly vulnerable group who often have multimorbidity and polypharmacy. In addition, geriatric syndromes, such as malnutrition, sarcopenia, and frailty, significantly affect the quality of life, which is already compromised by rheumatic diseases. Furthermore, the use of antirheumatic drugs (synthetic or biological) in this group of patients is a major concern due to their potential adverse events. The aim of this study is to Determine if drug discontinuation of bDMARDs differs in elderly patients compared to younger patients with rheumatic diseases registered in the Mexican Adverse Events Registry (BIOBADAMEX).
Methods: In this case-control study from an inception cohort from BIOBADAMEX we included all patients registered from 2016 to April 2023 with at least two assessments. We divided the patients in 2 groups: younger than 60 years and older than 60 years. Survival on similar or originator bDMARDs was estimated using Kaplan-Meier analysis. Predictors of discontinuation, including use of biosimilar drugs were investigated by Cox regression analysis.
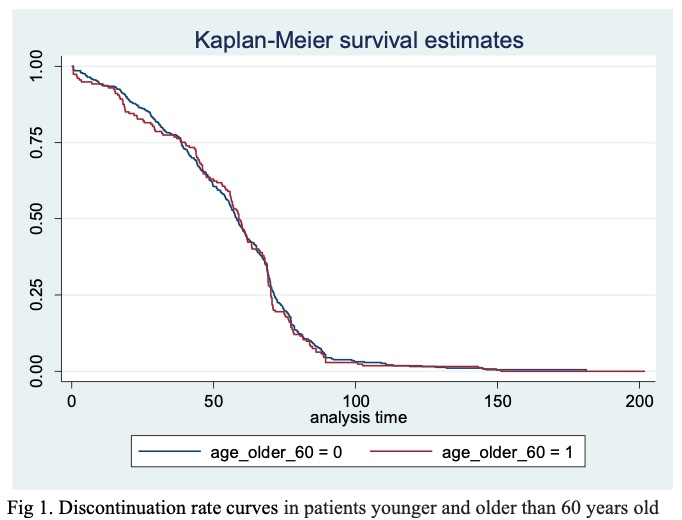
Results: Among 1041 patients in the registry, 630 had at least two assessments. Of patients analyzed, 128 (20.3%) were older than 60 years old and 512 (81.3%) were women. The most common diagnoses were RA in 407 (64.6%), ankylosing spondylitis in 83 (13.2%) patients and psoriatic arthritis in 44 (6.9%). At baseline, patients had a median (IQR) age of 50.5 (40.1-58.1) years old, median disease duration of 6.9 (2.7-13.9) years. The most common bDMARDs received were adalimumab 122 (19.4%), certolizumab 96 (15.2), tocilizumab 95 (15.1), abatacept 82 (13.0) and rituximab 67 (10.6). At the time of analysis, the median bDMARDs treatment duration was 58.8(38.6-70.7) months, 264 (41.9%) had discontinued treatment, 135 for inefficacy, 108 for adverse events and 21 for others. Fig 1 shows discontinuation rate curves in patients younger and older than 60 years old.Cox proportional-hazards demonstrated no significant differences regarding age older than 60 years old (HR 1.0, 95% CI 0.8-1.2, p=0.7), age as continuous variable (HR 0.9, 95% CI 0.9-1.0, p=0.07), use of corticosteroids (HR 1.2, 95% CI 0.9-1.4, p=0.05) or comorbidities (HR 0.9, 95% 0.6-1.5, p=0.78). To have a disease duration shorter than the median (≤6.9 years) (HR 1.2, 1.1-1.4 p=0.03), history of an adverse event (HR 2.4, 1.1-4.8, p=0.02) and high disease activity (HR 1.5,95%CI 1.2-1.7, p< 0.001) were characteristics associated with discontinuation. In the multivariable Cox regression analysis history of adverse event and high disease activity were the only variables that remained associated.
Conclusion: This analysis did not show a role of elderly age on discontinuation of bDMARDs in Mexican patients with rheumatic diseases. Survival analysis showed that in our population high disease activity and history of adverse events are associated with the discontinuation of bDMARDs.
To cite this abstract in AMA style:
Rivera Terán V, Vega Morales D, Saavedra Salinas M, Xibillé Friedman D, Colunga I, Carrillo Vazquez S, Miranda Hernández D, Durán Barragán S, Zamora Tehozol E, Castillo Ortiz A, Sicsik Ayala S, Irazoque Palazuelos F, Casasola Vargas J, Peña A, Muñoz Monroy O, Ramos Sánchez A, Valdés Corona L, Merayo-Chalico J, Torres Valdez E, Paz Viscarra A, Alpízar Rodríguez D. Elderly Patients’ Discontinuation of Biologic DMARDs in Patients with Rheumatic Diseases: Data from the Mexican Adverse Events Registry (BIOBADAMEX) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/elderly-patients-discontinuation-of-biologic-dmards-in-patients-with-rheumatic-diseases-data-from-the-mexican-adverse-events-registry-biobadamex/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/elderly-patients-discontinuation-of-biologic-dmards-in-patients-with-rheumatic-diseases-data-from-the-mexican-adverse-events-registry-biobadamex/