Session Information
Date: Monday, November 14, 2022
Title: Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Chronic kidney disease (CKD) has been associated with gout1 and CKD worsening has been associated with hyperuricemia.2,3 Pegloticase can lower serum uric acid (sUA) in uncontrolled gout patients but anti-drug antibodies have been linked to loss of urate-lowering response and high risk of infusion reaction (IR).4 The literature strongly supports using immunomodulators (IMMs) as co-therapy to pegloticase to increase the proportion of patients who maintain urate-lowering response5 and lower IR risk.6 However, CKD is common in gout patients and can limit IMM use (particularly methotrexate [MTX]). This study examined eGFR changes during pegloticase+MTX co-therapy in uncontrolled gout patients with and without CKD.
Methods: Cases of pegloticase+MTX co-therapy were extracted from existing deidentified data sets and retrospectively examined. Baseline eGFR was used to group patients into CKD (< 60 ml/min/1.73m2) and non-CKD (≥60 ml/min/1.73m2) cohorts. Laboratory values were monitored during treatment, including sUA, eGFR, blood cell counts, and liver function tests. Patient characteristics, treatment parameters, eGFR, and AEs were examined, along with rate of sustained urate-lowering response (defined as ≥12 infusions received and sUA < 6 mg/dL just prior to infusion 12). Patients who remained on therapy at the time of data collection and had not yet received 12 infusions were excluded from response rate analyses.
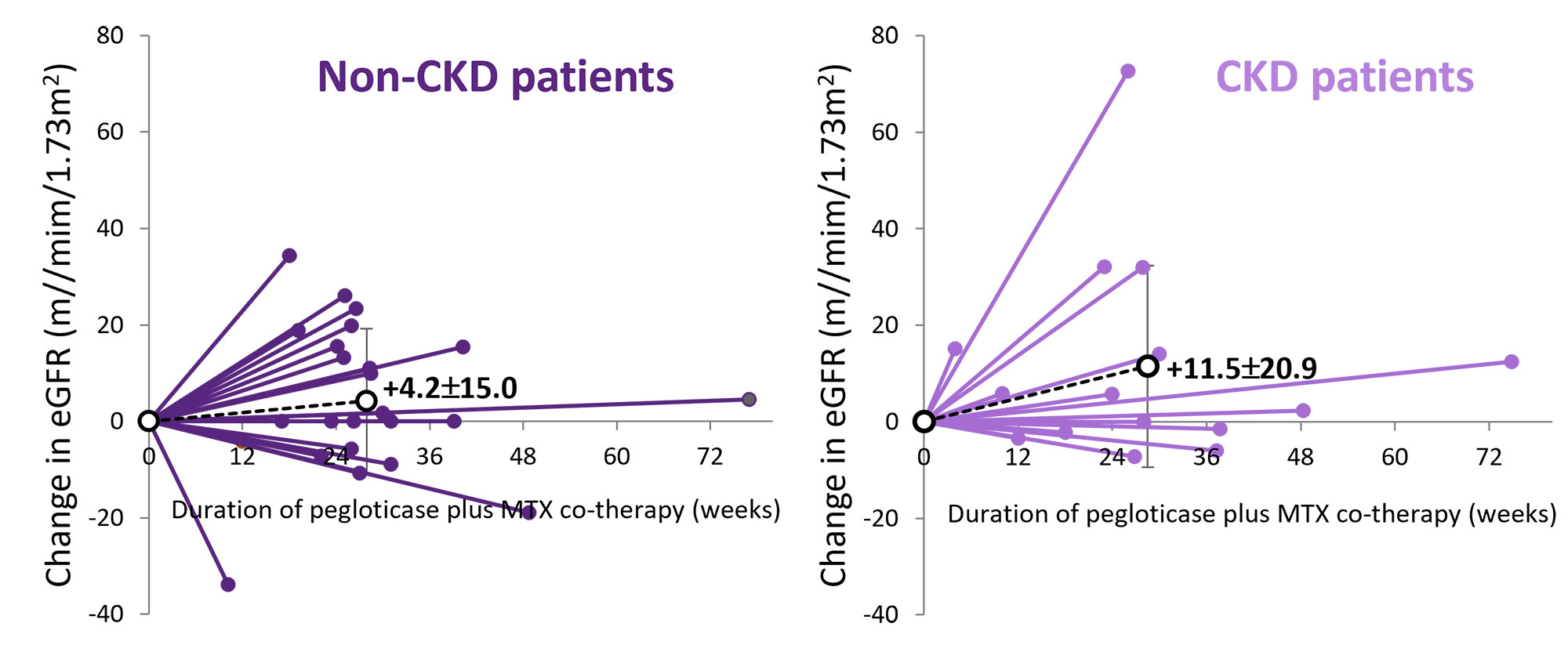
Results: 42 uncontrolled gout patient were included. 15 were classified as having CKD (13 stage 3 and 2 stage 4; baseline mean eGFR: 43±11 ml/min/1.73m2; sUA=8.6±2.2 mg/dL) and 27 as non-CKD (baseline mean eGFR: 83±19 ml/min/1.73m2; sUA=9.5±1.7 mg/dL). Comorbidity profiles were similar, but CKD patients were older (72.0±9.9 vs. 52.3±14.3 years, ≥65 years: 60% vs. 19%) and more often female (33% vs 7%). MTX was started approximately 4 weeks prior to first pegloticase infusion in both groups, but CKD patients had a lower mean MTX dose (14.8±5.8 vs. 19.3±4.9 mg/week). Number of pegloticase infusions (CKD: 14.7±8.1 vs. non-CKD 14.1±7.1 infusions) and sustained urate-lowering response rate (92% vs. 86%) was similar between groups. eGFR increased in 44% of non-CKD (mean change: +4.2±15.0 ml/min/1.73m2) and in 60% of CKD (+11.5±20.9 ml/min/1.73m2) patients and 13/15 CKD patients (87%) had stable or improved CKD stage (2 moved from stage 3a to 3b). 3 non-CKD patients moved from Stage 2 to 3b (n=2) or 3a (n=1). AEs were reported in 7 (47%) CKD and 13 (48%) non-CKD patients, with gout flare most common in both groups. Pancytopenia (n=1) and mild IR (n=1) were also reported in the non-CKD group.
Conclusion: These limited data show similar pegloticase+MTX urate-lowering efficacy in CKD and non-CKD patients. Additionally, no new safety signals were identified with the majority of CKD patients showing eGFR stability or improvement during therapy.
References
- Roughley MJ et al. Arthritis Res Ther 2015;17:90
- Edwards NL. Cleve Clin J Med 2008;75:S13-6
- Iseki K et al. Am J Kidney Dis 2004;44:642-50
- Sundy et al. JAMA 2011;306:711-20
- Keenan RT et al. Semin Arthritis Rheum 2021;51:347-52
- Peterson J et al. Semin Arthritis Rheum 2021;51:1386-88
To cite this abstract in AMA style:
Albert J, Broadwell A, Padnick-Silver L, Marder B, LaMoreaux B. eGFR Changes in Uncontrolled Gout Patients Undergoing Pegloticase + Methotrexate Co-therapy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/egfr-changes-in-uncontrolled-gout-patients-undergoing-pegloticase-methotrexate-co-therapy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/egfr-changes-in-uncontrolled-gout-patients-undergoing-pegloticase-methotrexate-co-therapy/