Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Rheumatoid arthritis (RA) patients (pts) with inadequate response or intolerance to current biologic/targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs) have limited options. Abiprubart, a humanized IgG4 monoclonal antibody with a stabilized/functionally-silent Fc, binds CD40 and inhibits CD40/CD154 costimulation without lymphocyte depletion. Abiprubart was well-tolerated and showed sustained target engagement and T-cell dependent antibody response suppression in phase 1. These data and abiprubart’s high-concentration liquid formulation support investigation of chronic subcutaneous (SC) administration in autoimmune diseases.
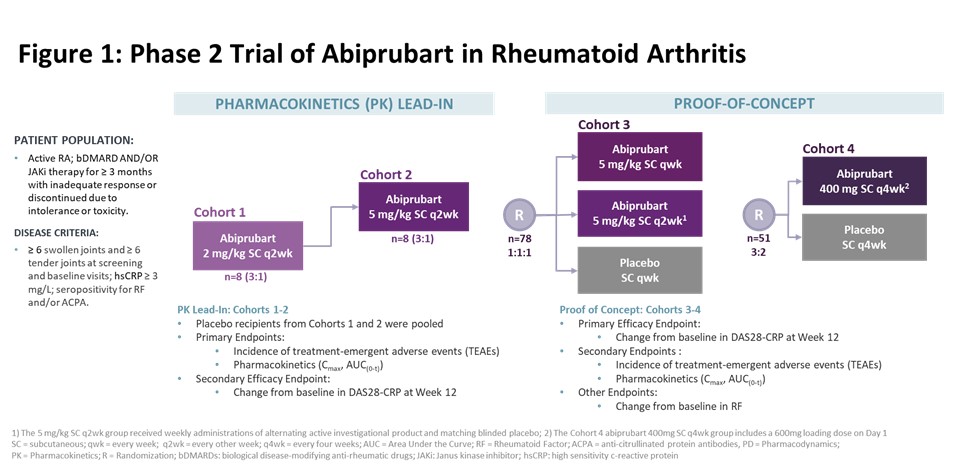
Methods: This phase 2, randomized, double-blind, placebo (PBO)-controlled study enrolled [adult] pts with moderate-to-severe active RA with inadequate response or intolerance to ≥1 bDMARD or Janus kinase inhibitor. Participants were randomized in sequential cohorts to 12-week (wk) treatment [Fig 1]. Cohorts 1 & 2 measured safety and PK. Primary efficacy endpoint in Cohorts 3 & 4 was change from baseline (BL) in Disease Activity Score of 28 Joints using C-Reactive Protein (DAS28-CRP) at Wk 12. Change from BL at Wk 12 in Rheumatoid Factor (RF) was assessed. Efficacy comparisons used an analysis of covariance model comparing abiprubart groups vs PBO; BL value and randomization stratification as covariates.
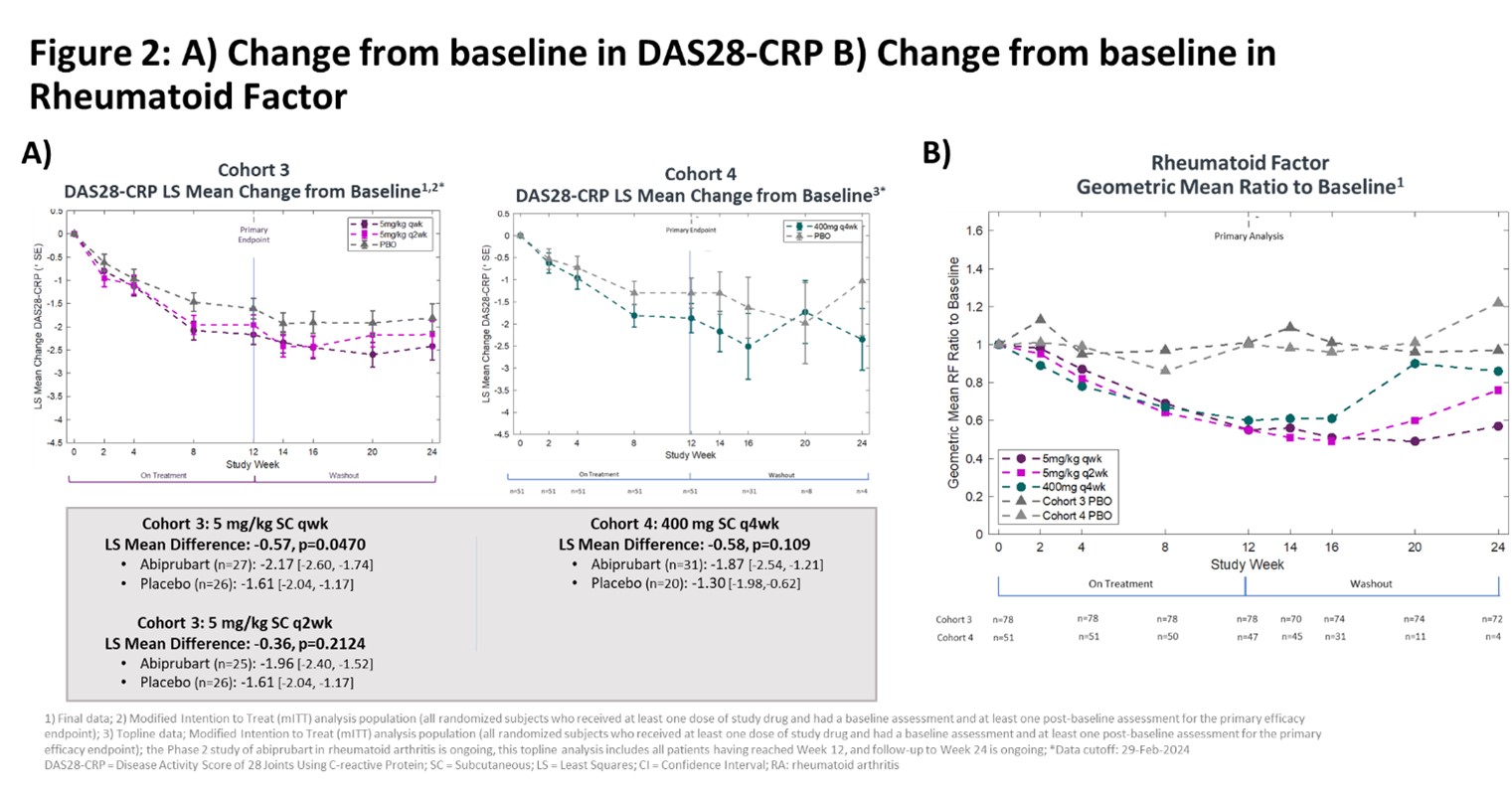
Results: In Cohorts 1 & 2 (n=16) and Cohorts 3 & 4 (n=129) demographics and BL disease activity were balanced. Abiprubart was well tolerated in Cohorts 1 & 2, enabling Cohort 3. In Cohort 3, the least-squares (LS) mean change [95% confidence interval] from BL in DAS28-CRP at Wk 12 in the 5 mg/kg SC qwk group (‑2.17 [‑2.60, ‑1.74], n=27) compared to PBO (‑1.61 [‑2.04, ‑1.17], n=26) was statistically significant (‑0.57, p=0.0470). In the 5 mg/kg SC q2wk group, the improvement in LS mean change from BL in DAS28-CRP at Wk 12 (‑1.96 [‑2.40, ‑1.52], n=25) vs PBO (‑1.61 [‑2.04, ‑1.17], n=26) did not reach statistical significance (‑0.36, p=0.2124) [Fig 2A]. In Cohort 4, LS mean change from BL in DAS28-CRP at Wk 12 was ‑1.87 [‑2.54, ‑1.21] for abiprubart (400 mg SC q4wk; n=31) vs ‑1.30 [‑1.98, ‑0.62] in PBO (n=20) (‑0.58, p=0.109). Efficacy curves continued to separate until Wk 16 despite treatment cessation at Wk 12. Abiprubart significantly reduced RF (pharmacodynamic marker of CD40 target engagement) by >40% [Fig 2B]. During follow-up, RF levels returned to BL inversely to dose frequency. In a pooled (Cohorts 3 & 4) post-hoc analysis, LS mean change from BL in DAS28-CRP at Wk 12 for abiprubart (‑2.04 [‑2.34, ‑1.74], n=83) vs combined PBO (‑1.52 [‑1.88, ‑1.16], n=46) recipients was statistically significant (-0.52, nominal p=0.010). TEAEs were similar across groups, without dose relatedness; all mild or moderate in severity.
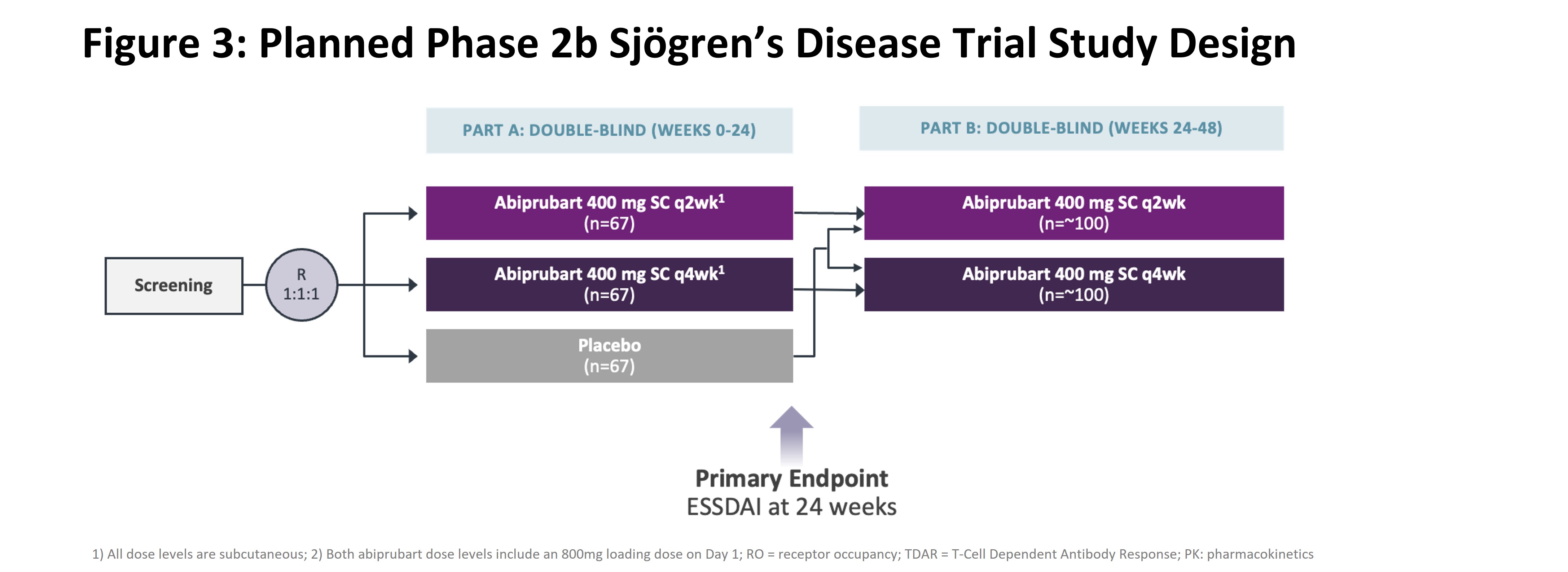
Conclusion: Abiprubart was well-tolerated in pts with b/tsDMARD-refractory RA, and reductions in DAS28-CRP at Wk 12 for all abiprubart groups were greater than PBO, with statistical significance in the 5mg/kg SC qwk dose group and pooled analysis. Abiprubart may provide benefit in autoimmune diseases involving CD40/CD154 costimulation. A phase 2B study [Fig 3] in Sjögren’s Disease is planned.
To cite this abstract in AMA style:
Jenkins E, Louw I, Balog A, van Duuren E, Horowitz d, Jaworski J, Kivitz A, Kemplerne Ujfalussy I, Pirrello J, Tessari E, Wang S, Paolini J. Efficacy, Safety, Pharmacokinetics of Anti-CD40 Antibody Abiprubart in Patients with Rheumatoid Arthritis: A Phase 2, Randomized, Placebo-Controlled 12-week-treatment Proof-of-Concept Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-safety-pharmacokinetics-of-anti-cd40-antibody-abiprubart-in-patients-with-rheumatoid-arthritis-a-phase-2-randomized-placebo-controlled-12-week-treatment-proof-of-concept-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-safety-pharmacokinetics-of-anti-cd40-antibody-abiprubart-in-patients-with-rheumatoid-arthritis-a-phase-2-randomized-placebo-controlled-12-week-treatment-proof-of-concept-study/