Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Interleukin (IL)-10 is a regulator of cytokine activity and has the potential to restore homeostasis in osteoarthritis (OA) of the knee. Unfortunately, relatively short half-life has limited the therapeutic potential of native IL-10 protein.1 The primary objective of this study (NCT04124042) was to establish the safety and tolerability of a single intra-articular (IA) dose of XT-150, a non-viral gene therapy designed to transiently express a proprietary version of the anti-inflammatory cytokine IL-10. The secondary objective was to establish the analgesic efficacy of a single IA dose of XT-150. Exploratory analyses evaluated effects on pain and function following single and repeat doses.
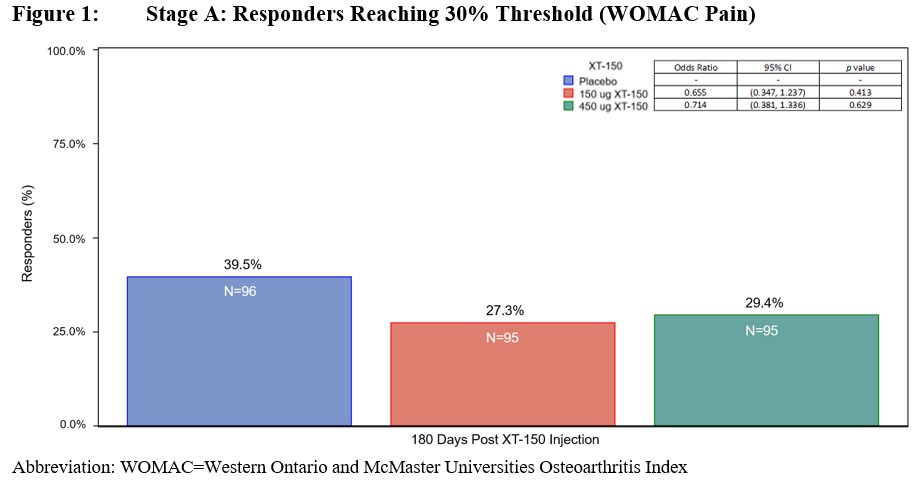
Methods: This was a two-stage double-blind Phase 2 study. Stage A compared 2 active doses of XT-150 to a placebo control arm. Stage B was a 6-month follow-up with the option to randomly receive a single injection of XT-150 at one of two doses (0.15 or 0.45 mg) between Day 180 and Day 330. The dose range was chosen from supportive evidence in previous clinical (XT-150-1-0201 & XT-150-1-0202) and preclinical studies. Participants 45-85 years of age with symptomatic knee OA (defined as a WOMAC Pain score of ≥8 at screening and Kellgren-Lawrence grade 2 or 3) were randomized on Day 0 to one of 6 treatment sequences. Injections were completed under imaging guidance to confirm positioning within the joint prior to injection. Screening evaluation took place up to 30 days before enrollment. Efficacy measures were evaluated at baseline and at follow-up visits on Day 7, 30, and monthly thereafter. In Stage A, 286 participants received at least one dose of XT-150 or placebo (phosphate buffered saline) as a single IA injection into the knee. In Stage B, 244 received a second injection, all with XT-150. Separate analyses were conducted for Stages A and B. Stage A analyses included the primary intent to treat (ITT) population to assess the efficacy of XT-150. The primary efficacy outcome measure was at least 30% improvement from Baseline in WOMAC Pain score (obtained from KOOS questionnaire) at Day 180. Stage B analyses included a post hoc modified ITT (mITT) population with a baseline WOMAC Pain score of 9-20. Post hoc analyses in the ITT and mITT populations compared single active doses with repeat active doses.
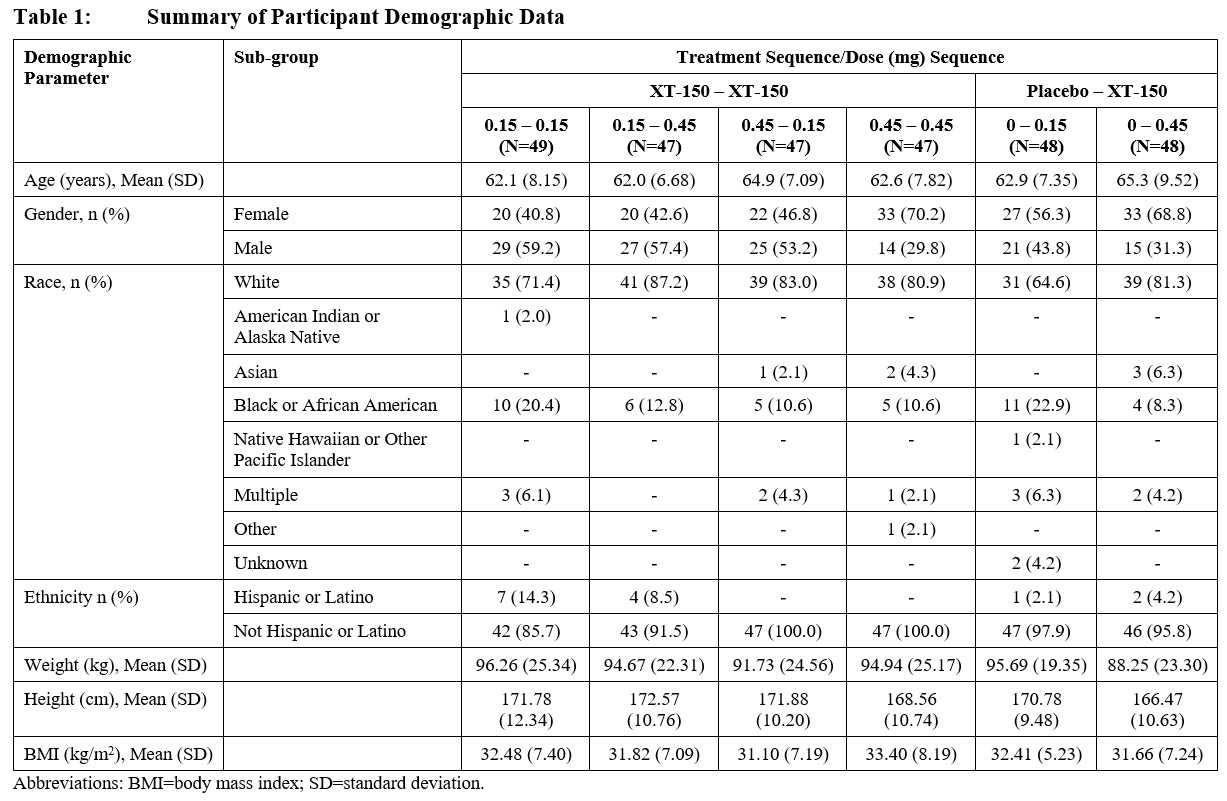
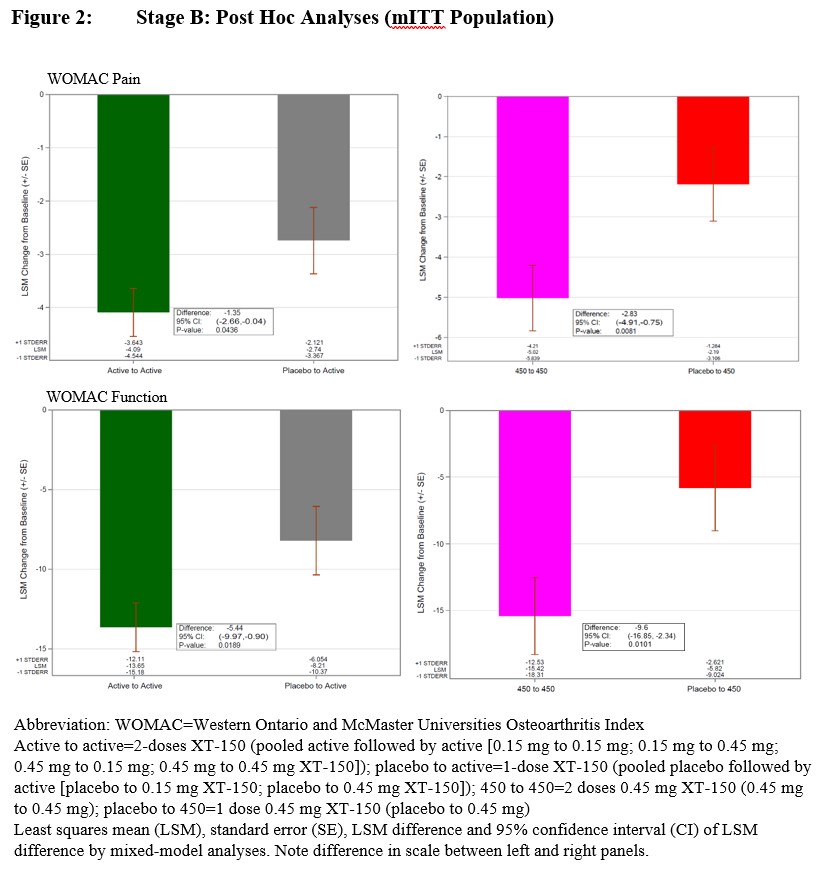
Results: Demographic data are summarized in Table 1. There was no statistically significant difference in patients achieving at least 30% improvement in WOMAC Pain score between either dose (0.15 mg or 0.45 mg) of XT-150 vs placebo when given as a single dose by Day 180 (Figure 1). Statistically significant changes from baseline WOMAC Pain scores were observed at Day 360 comparing 2 doses of 0.45 mg XT-150 vs 1 dose of placebo followed by 1 dose of 0.45 mg XT-150 in post hoc analyses (p=0.0081, Figure 2). The two-dose regimen of 0.45 mg XT-150 also showed a statistically significant reduction in WOMAC function score at Day 360 vs the single-dose regimen of 0.45 mg XT-150 (p=0.0101).
Conclusion: These data suggest improvement in pain and function with two doses vs one dose of XT-150 and support the future development of repeat doses of XT-150 for the potential treatment of pain from moderate-to-severe knee OA.
1. Kwilasz AJ, et al. Neuropharmacology. 2015;96(Pt A):55-69.
To cite this abstract in AMA style:
Grigsby E, Collins S, Kapural L, McBride M, Rieger J, Stokes M, Rutman H, Cicuttini F. Efficacy of XT-150, a Novel Non-Viral Gene Therapy Delivering IL-10v, on Moderate to Severe Pain Due to Osteoarthritis of the Knee: Results of a Phase 2 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-xt-150-a-novel-non-viral-gene-therapy-delivering-il-10v-on-moderate-to-severe-pain-due-to-osteoarthritis-of-the-knee-results-of-a-phase-2-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-xt-150-a-novel-non-viral-gene-therapy-delivering-il-10v-on-moderate-to-severe-pain-due-to-osteoarthritis-of-the-knee-results-of-a-phase-2-trial/