Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Ankylosing spondylitis (AS) is a chronic inflammatory disease that primarily affects the spine and sacroiliac joints, resulting in significant pain, stiffness, and functional impairment. The interleukin-17 (IL-17) signaling pathway plays a central role in the pathogenesis of AS. Vunakizumab, a humanized monoclonal antibody targeting IL-17A, has demonstrated clinical efficacy in a randomized, double-blind, phase 2/3 trial. Since pain is the most common and serious symptom experienced by patients with AS, it is very important to develop and use effective treatment strategies to manage it. This post-hoc analysis aimed to evaluate the efficacy of vunakizumab in reducing pain outcomes in patients with active AS.
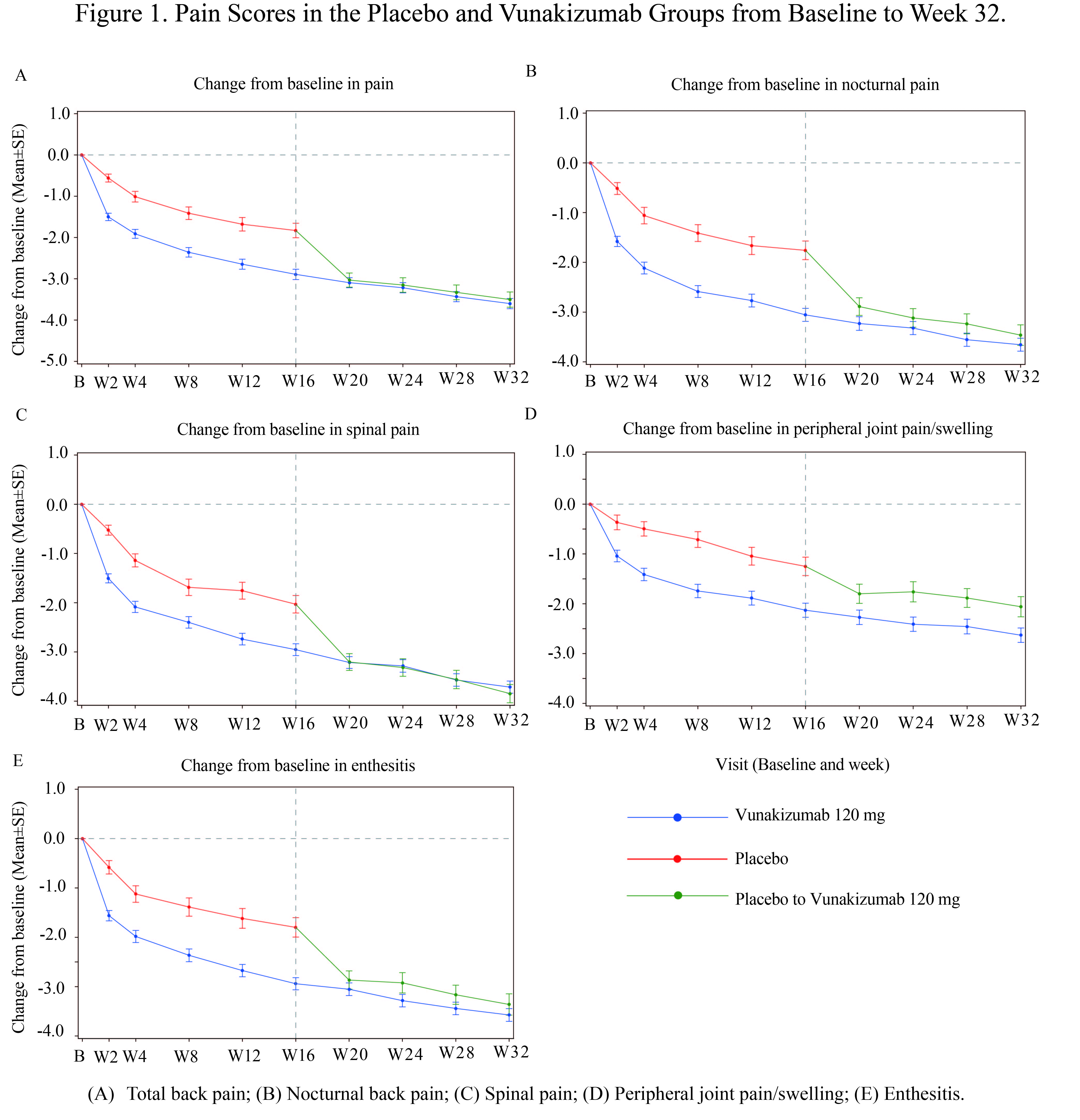
Methods: Eligible participants were adults with active AS, diagnosed according to the modified New York criteria (1984 revision), who had an inadequate response to, intolerance of, or contraindications to non-steroidal anti-inflammatory drugs (NSAIDs). All patients included in this study fulfilled the American College of Rheumatology (ACR) classification criteria. In phase 2, patients were randomized in a 2:2:1 ratio to receive either 120 mg or 240 mg of vunakizumab, or placebo. At week 16, patients initially receiving placebo were re-randomized 1:1 to one of the treatment groups. In phase 3, patients were randomized in a 2:1 ratio to receive 120 mg of vunakizumab or placebo. Study drug was administered at weeks 0, 2, 4, 8, and 12, followed by dosing every four weeks through week 32. Pain outcomes included total back pain, nocturnal back pain, and questions 2–4 from the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI): spinal pain (pain in the back, neck, or hip), peripheral joint pain/swelling, and enthesitis (pain in areas tender to touch).
Results: This post-hoc analysis demonstrated significant reductions in all five pain outcome scores for patients receiving vunakizumab 120 mg compared to placebo over the 16-week period (all nominal p < 0.0001 by repeated measures mixed-effects model; Figure 1). Specifically, at week 16, the vunakizumab group showed a mean reduction in total back pain of -2.89 versus -1.83 in the placebo group (nominal p < 0.0001). Similar significant improvements were observed in nocturnal back pain (-3.06 vs. -1.76, nominal p < 0.0001), spinal pain (-2.95 vs. -2.03, nominal p < 0.0001), peripheral joint pain/swelling (-2.13 vs. -1.25, nominal p = 0.0001), and enthesitis (-2.94 vs. -1.80, nominal p < 0.0001) (Table 1). From weeks 16 to 32, patients initially assigned to placebo who switched to vunakizumab 120 mg at week 16 experienced accelerated reductions in all five pain outcomes after the switch, although the magnitude of improvement remained slightly less than that observed in patients who received continuous vunakizumab throughout the study.
Conclusion: Vunakizumab provided statistically significant and clinically meaningful reductions in pain outcomes in patients with active AS. Importantly, patients who switched from placebo to vunakizumab at week 16 experienced rapid pain improvement, supporting vunakizumab as an effective treatment strategy for managing pain in AS.
To cite this abstract in AMA style:
Xu J, Yang Y, Liu S, Bai R, Li S, Zhang G, Wang X, Xu X, Zheng M, Feng D. Efficacy of Vunakizumab in Reducing Pain in Ankylosing Spondylitis: A Post-Hoc Analysis of a Phase 2/3 Clinical Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-vunakizumab-in-reducing-pain-in-ankylosing-spondylitis-a-post-hoc-analysis-of-a-phase-2-3-clinical-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-vunakizumab-in-reducing-pain-in-ankylosing-spondylitis-a-post-hoc-analysis-of-a-phase-2-3-clinical-trial/

.jpg)