Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Involvement of weight-bearing joints in patients with PsA can be associated with reduced activities of daily living and quality of life. Upadacitinib (UPA) is an oral JAK inhibitor approved for the treatment of active PsA in adults. In the SELECT-PsA 1 and SELECT‑PsA 2 trials, treatment with once daily UPA 15 mg (UPA15) or 30 mg (UPA30) resulted in greater improvements in the number of swollen and tender joints as well as other patient- and physician-reported outcomes when compared with placebo.1–3 The objective of this post hoc analysis was to evaluate the long-term efficacy of UPA in patients with PsA and involvement in 1 or more regions of weight-bearing joints.
Methods: The SELECT-PsA 1 and SELECT-PsA 2 phase 3, randomized, controlled trials enrolled patients ≥ 18 years old with active PsA and intolerance or inadequate response to ≥ 1 nonbiologic disease modifying anti-rheumatic drug (DMARD; SELECT-PsA 1) or biologic DMARD (SELECT-PsA 2). This post hoc analysis of the 2 phase 3 trials stratified patients with involvement in 1 to 6 regions of weight-bearing joints (hips, knees, ankles, midfoot, metatarsophalangeal [MTP], or proximal interphalangeal [PIP]) who were randomized at baseline to receive either UPA15, placebo (PBO), or adalimumab (ADA; only in SELECT-PsA 1); at week 24, PBO‑treated patients switched to UPA15 (PBO/UPA15). This analysis did not include patients who received UPA30 or PBO/UPA30. A mixed-effects model with repeated measures was used to analyze the change from baseline to week 104 in 4 patient-reported outcome measures (pain, Health Assessment Questionnaire-Disability Index [HAQ-DI], Work Productivity and Activity Impairment [WPAI] questionnaire, and EuroQol 5 Dimension 5 Level [EQ-5D-5L]) and 1 composite outcome measure (Disease Activity in Psoriatic Arthritis [DAPSA]). UPA safety results in patients with PsA have been previously reported.1–3
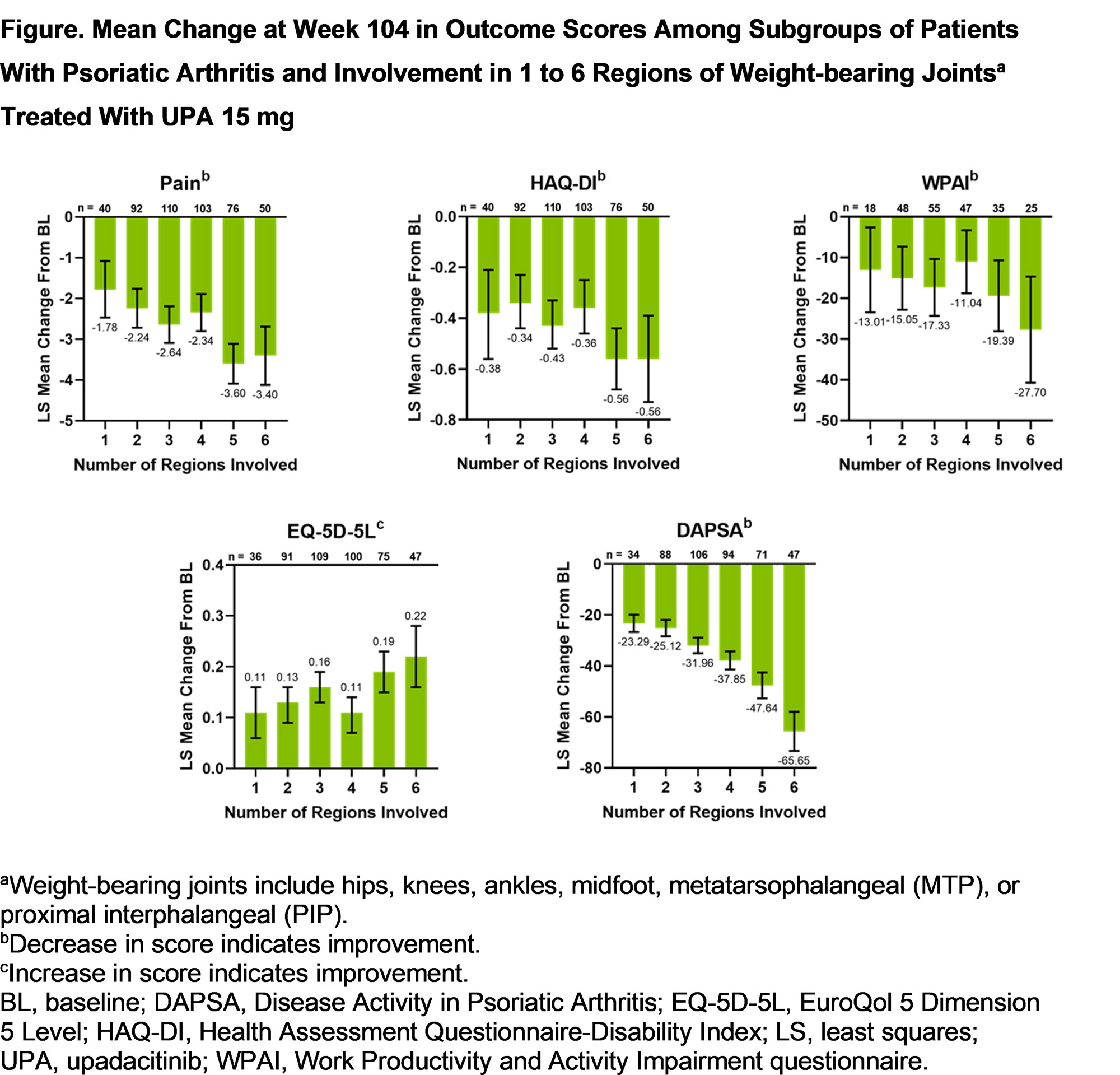
Results: A total of 1069 patients from the SELECT-PsA 1 trial and 317 patients from the SELECT-PsA 2 trial were included in the pooled analysis. Of all patients with any involvement in regions of weight-bearing joints, > 91% of patients had involvement in ≥ 2 regions. The most common weight-bearing joints with involvement were the PIP (SELECT-PsA 1, 79.8%; SELECT-PsA 2, 84.5%), MTP (71.1%; 73.8%), and knee (62.3%; 64.9%) joints. Improvements from baseline to week 104 were observed with UPA15 treatment in patient-reported outcomes of pain, HAQ-DI, WPAI, EQ-5D-5L, and the DAPSA score across all subgroups of patients with involvement in 1 to 6 regions of weight-bearing joints (Figure). In general, the magnitude of improvement in outcome scores with UPA15 was greater in patients with involvement in a higher number of regions. Similar improvements from baseline to week 104 in outcome scores were observed in patients who received PBO/UPA15 or ADA to week 104.
Conclusion: UPA15 treatment was associated with long-term improvements in pain, disability, work productivity, disease activity, and quality of life in patients with PsA and involvement in 1 or more regions of weight-bearing joints.
References:
- McInnes IB, et al. N Engl J Med. 2021;384;1227-39.
- McInnes IB, et al. RMD Open. 2021;7:e001838.
- Mease PJ, et al. Ann Rheum Dis. 2022;80:312-20.
To cite this abstract in AMA style:
Mizelle K, Tillett W, Ali M, Iyile T, Gao T, Setty A, Walsh J, Coates L. Efficacy of Upadacitinib in Patients with Psoriatic Arthritis Stratified by Involvement of Weight-bearing Joint Regions: A Post Hoc Subgroup Analysis of the Phase 3, Randomized, SELECT-PsA 1 and SELECT-PsA 2 Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-psoriatic-arthritis-stratified-by-involvement-of-weight-bearing-joint-regions-a-post-hoc-subgroup-analysis-of-the-phase-3-randomized-select-psa-1-and-selec/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-psoriatic-arthritis-stratified-by-involvement-of-weight-bearing-joint-regions-a-post-hoc-subgroup-analysis-of-the-phase-3-randomized-select-psa-1-and-selec/