Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The phase 3 randomized controlled trial FUTURE 5 (NCT02404350) showed the efficacy of secukinumab (SEC) improving clinical signs, symptoms, and radiographic progression in patients with active psoriatic arthritis (PsA).1 This post hoc analysis evaluated the efficacy of SEC improving patient-reported outcomes (PROs) in tumor necrosis factor inhibitor (TNFi)-naive and inadequate-responder (TNFi-IR) patients with PsA.
Methods: FUTURE 5 enrolled patients ≥ 18 years who met CASPAR with symptoms for ≥ 6 months at screening. Patients were randomized to receive SEC 300 mg, 150 mg, 150 mg no load (NL), or placebo (PBO) weekly from baseline to week 4 and every 4 weeks thereafter to week 16. Patients in the SEC 150 mg NL arm received PBO at weeks 1, 2, and 3. For this post hoc analysis, patients were stratified by prior TNFi use. PROs were assessed at baseline and selected visits through week 16. Mean changes from baseline in PROs and the proportions of patients reporting improvements ≥ minimum clinically important differences (MCIDs) and scores ≥ age/gender-matched normative values were assessed in each treatment group. PROs included patient global assessments of disease activity (PtGA) and psoriasis and arthritis (PtGA PsO/arthritis) visual analog scale (VAS), pain VAS, Health Assessment Questionnaire Disability Index (HAQ-DI), 36-item Short Form Health Survey (SF-36), Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue, and Dermatology Life Quality Index (DLQI; patients with psoriasis only).
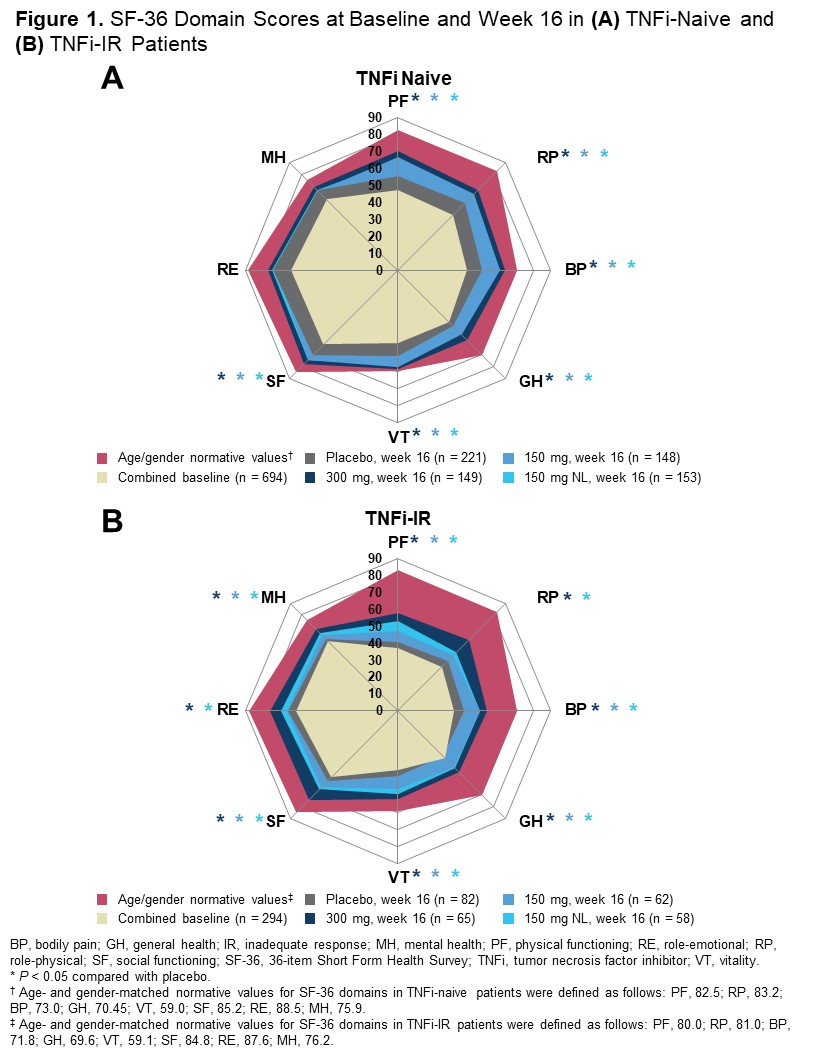
Results: Baseline PRO scores were generally worse in the TNFi-IR vs TNFi-naive population. TNFi-naive and TNFi-IR patients in all SEC treatment groups reported significantly greater improvements in PtGA, PtGA PsO/arthritis, pain, HAQ-DI, SF-36 physical component summary (PCS), and DLQI at week 16 vs PBO. Treatment responses were higher in the SEC 300-mg arm vs SEC 150-mg and 150-mg NL arms at week 16 across most PROs, including all SF-36 domains (Figure 1). Significantly higher proportions of both TNFi-naive and TNFi-IR patients receiving SEC reported improvements ≥ MCID as early as week 1 in HAQ-DI and at week 16 across all PROs except SF-36 mental component summary score vs PBO. The proportions of patients reporting improvement ≥ MCID in pain and HAQ-DI (Figure 2), as well as PtGA PsO/arthritis, SF-36 PCS, and DLQI, were higher in TNFi-naive than TNFi-IR patients. More SEC-treated patients reported scores ≥ normative values in HAQ-DI, SF-36 PCS, and FACIT-Fatigue at week 16 vs PBO, with more patients in the TNFi-naive than the TNFi-IR sub-population reporting scores ≥ normative values (Figure 3).
Conclusion: Initiation of SEC as a first-line biologic in patients with PsA resulted in early, statistically significant, and clinically meaningful improvements in PROs across all doses, and significant and meaningful improvements in TNFi-IR patients as later-line therapy. Treatment responses were higher in patients receiving SEC 300 mg vs 150 mg or 150 mg NL. TNFi-naive patients more frequently reported improvements ≥ MCID and scores ≥ normative values vs TNFi-IR patients, although TNFi-IR patients had lower baseline PRO scores and reported lower PBO responses.
Reference:
1. Mease P, et al. Ann Rheum Dis. 2018;77:890-7.
To cite this abstract in AMA style:
Strand V, Kaeley G, Bergman M, Gladman D, Coates L, Hur P, Kim N, Parikh B, Pertel P, Mease P. Efficacy of Secukinumab on Patient-Reported Outcomes in Patients with Active Psoriatic Arthritis Stratified by Prior Tumor Necrosis Factor Inhibitor Use: Post Hoc Analysis from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-secukinumab-on-patient-reported-outcomes-in-patients-with-active-psoriatic-arthritis-stratified-by-prior-tumor-necrosis-factor-inhibitor-use-post-hoc-analysis-from-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-secukinumab-on-patient-reported-outcomes-in-patients-with-active-psoriatic-arthritis-stratified-by-prior-tumor-necrosis-factor-inhibitor-use-post-hoc-analysis-from-a-phase-3-trial/