Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Secukinumab is an IL-17 inhibitor approved to treat axial spondyloarthritis (axSpA), including radiographic (r)-axSpA and non-radiographic (nr)-axSpA. Recent ASAS-EULAR recommendations highlight the importance of individualized treatment of patients with axSpA according to symptoms, extra-musculoskeletal manifestations, and comorbidities. These diverse factors may be more prominent in older patients and potentially impact treatment outcomes. This post hoc analysis compared clinical responses to secukinumab treatment through Week 16 between older and younger patients with r-axSpA and nr-axSpA.
Methods: In this post hoc analysis, patients with r-axSpA were pooled from the MEASURE 1-5 trials (NCT01358175, NCT01649375, NCT02008916, NCT02159053, and NCT02896127), and patients with nr-axSpA were included from the PREVENT trial (NCT02696031). Patient subgroups were defined based on the median age at baseline (≥40 years vs 18 to < 40 years). All patients were randomized to secukinumab 150 mg, secukinumab 300 mg, or placebo through Week 16; clinical and MRI outcomes were assessed at Week 16. Treatment effect (secukinumab 150/300 mg vs placebo) within each age subgroup and the difference in treatment effect between age subgroups were assessed for binary clinical outcomes (listed in Table 1) by logistic regression using non-responder imputation. For change from baseline in continuous outcomes (listed in Table 2), the difference in treatment effect between age subgroups was assessed using mixed model repeated measures. For change from baseline in MRI measures of inflammation (listed in Table 3), the analysis of covariance (ANCOVA) model was applied. The younger patient group was used as a reference category. All P values were nominal.
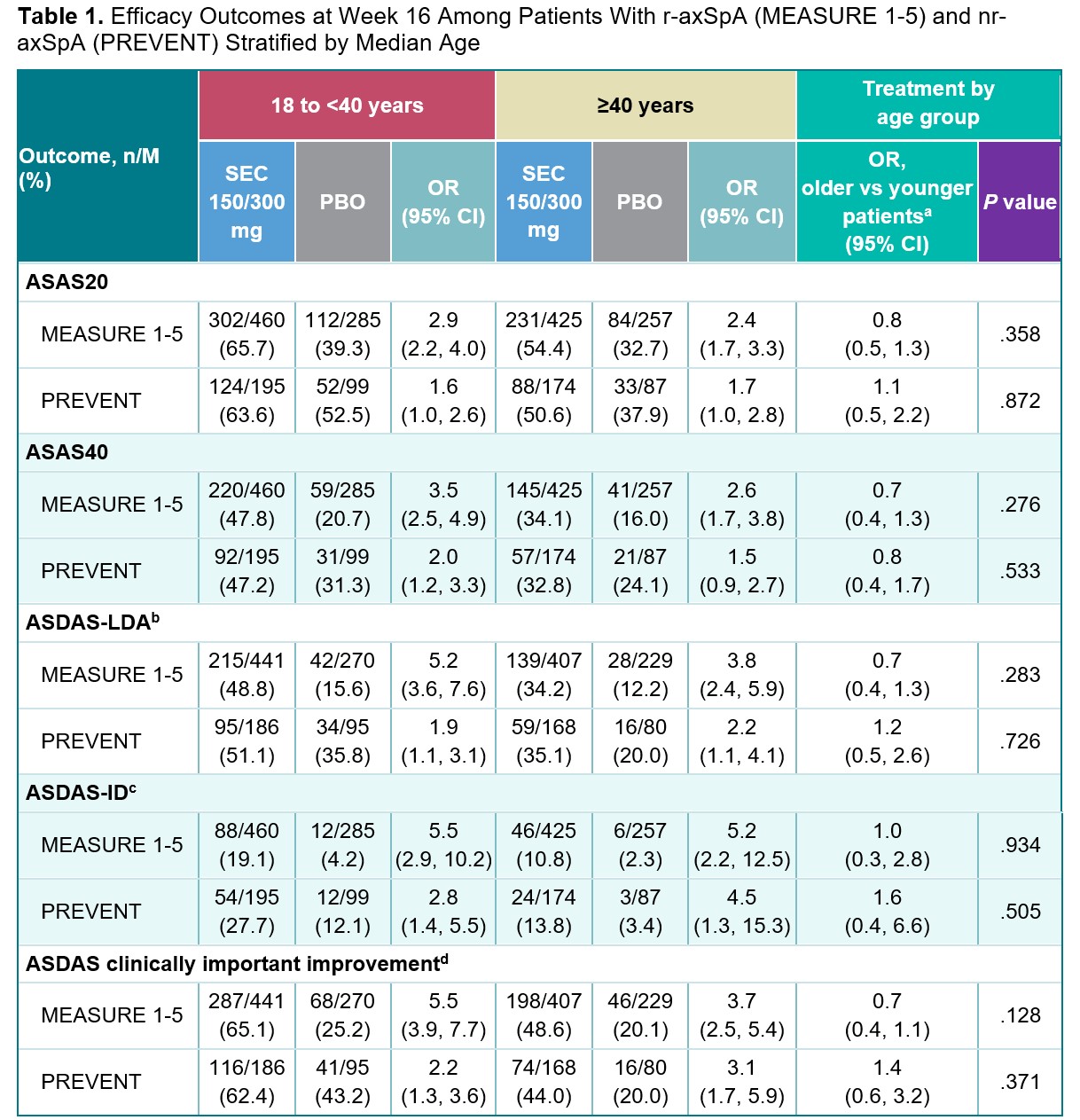
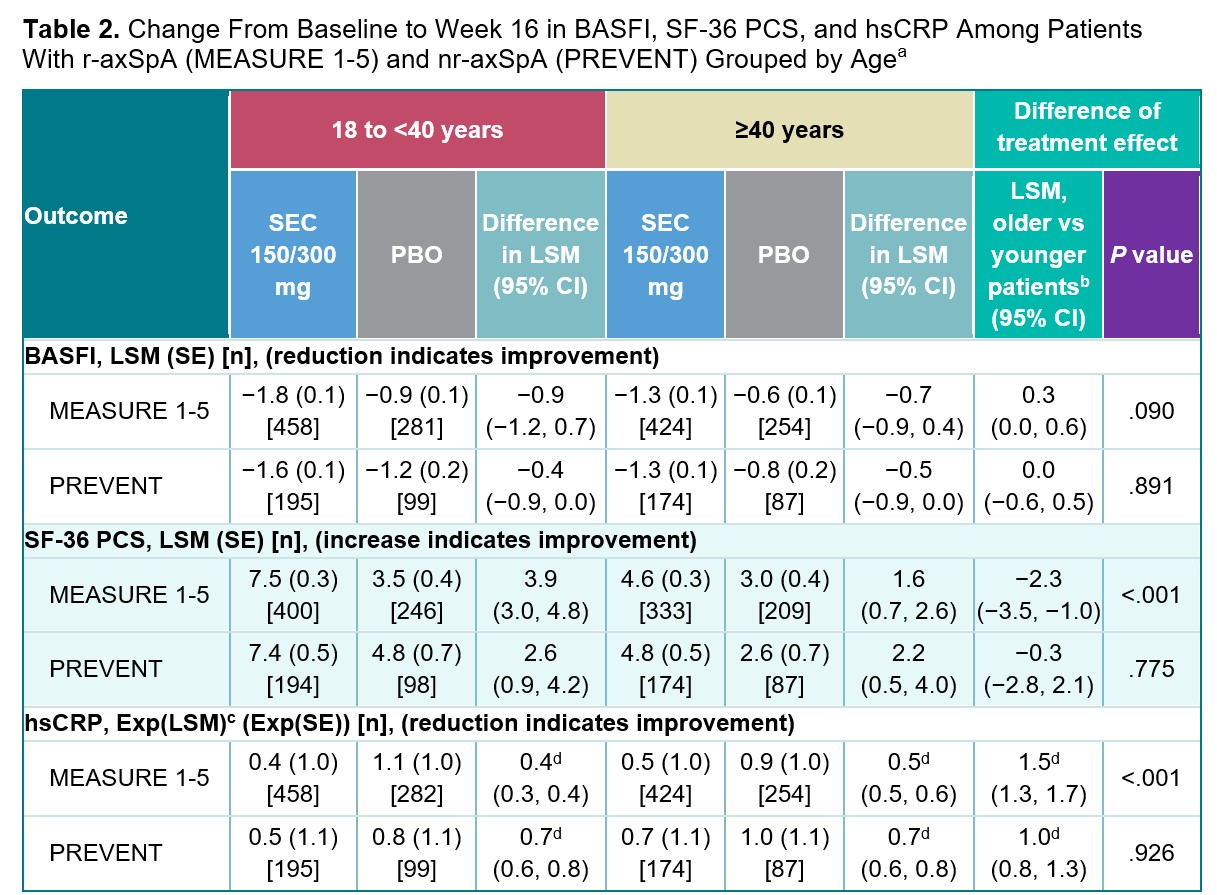
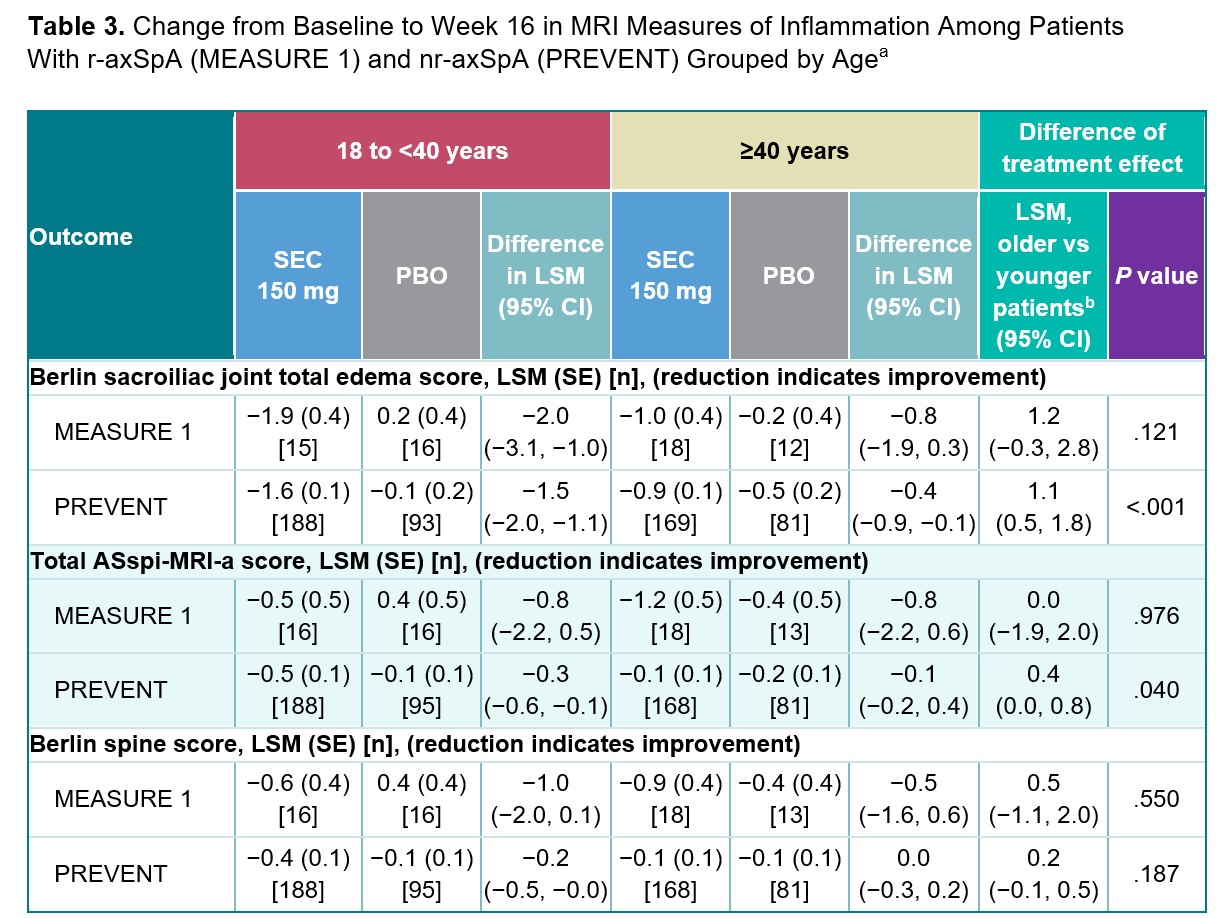
Results: Among 1427 patients from the MEASURE 1-5 trials and 555 from PREVENT, 682 (47.8%) and 261 (47.0%) were aged ≥40 years, and 745 (52.2%) and 294 (53.0%) were aged 18 to < 40 years, respectively. At Week 16, no difference in treatment effect of secukinumab vs placebo was observed between age groups for the achievement of binary clinical outcomes (ORs, P>.05; Table 1). No difference in treatment effect of secukinumab vs placebo was observed between age groups for BASFI improvement at Week 16 (P>.05; Table 2). Among patients with r-axSpA, older patients experienced a smaller treatment effect of secukinumab on the 36-Item Short Form Health Survey physical component score and high-sensitivity CRP than younger patients (P< .05; Table 2). Secukinumab led to improvements in MRI measures of inflammation in the sacroiliac joints and spine regardless of age group (Table 3); however, older patients with nr-axSpA experienced a smaller secukinumab treatment effect on the Berlin sacroiliac joint edema and ASspi-MRI-a scores than younger patients with nr-axSpA (P< .05).
Conclusion: Clinical efficacy of secukinumab was similar between age groups at Week 16 for patients with either r-axSpA or nr-axSpA. However, among patients with r-axSpA, less pronounced improvements in health-related quality of life and systemic inflammation were observed in the older age group. Older patients with nr-axSpA achieved smaller improvements in some MRI measures of axial inflammation than younger patients.
Missing responses for any reason are imputed as non-responders.
a The younger patient group was used as the reference category for the treatment effect comparison by age group; OR >1 indicates a larger treatment effect among older patients; OR <1 indicates a smaller treatment effect among older patients.
b ASDAS-LDA is defined as ASDAS of <2.1.
c ASDAS-ID is defined as ASDAS of <1.3.
d ASDAS clinically important improvement is defined as ASDAS improvement of ≥1.1.
a The difference in treatment effect between age subgroups was assessed using MMRM.
b The younger patient group was used as the reference category for the treatment effect comparison by age group; for BASFI, a positive value indicates a smaller treatment effect among older patients; for SF_36 PCS, a negative value indicates a smaller treatment effect among older patients; for hsCRP, a value >1 indicates a smaller treatment effect among older patients.
c Exponentially transformed LSM, the geometric mean ratio of postbaseline/baseline. A value of <1 indicates a reduced CRP.
d Relative treatment effect; exponential of the difference in LSM on the log(e) scale.
a The ANCOVA model was applied.
b The younger patient group was used as the reference category for the treatment effect comparison by age group; a positive value indicates a smaller treatment effect among older patients.
To cite this abstract in AMA style:
Ramiro S, Gaillez C, Kiltz U, Gensler L, Pisal C, Braun J, Ogdie A. Efficacy of Secukinumab Across the Axial Spondyloarthritis Spectrum Among Patients Grouped by Age (≥40 Years vs < 40): A PostHoc Analysis of 6 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-secukinumab-across-the-axial-spondyloarthritis-spectrum-among-patients-grouped-by-age-%e2%89%a540-years-vs-40-a-posthoc-analysis-of-6-phase-3-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-secukinumab-across-the-axial-spondyloarthritis-spectrum-among-patients-grouped-by-age-%e2%89%a540-years-vs-40-a-posthoc-analysis-of-6-phase-3-trials/