Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: ASCERTAIN (NCT01768572) was a 24-week, randomized, double-blind, double-dummy, parallel-group, 3-arm, safety study in patients with RA and inadequate response to or intolerance of TNF inhibitors receiving background conventional synthetic (cs)DMARDs. The purpose of this post hoc analysis was to examine outcomes for patients completing ASCERTAIN who were switched to sarilumab 200 mg every 2 weeks (q2w) subcutaneously (SC) in an open-label extension study (EXTEND, NCT01146652).
Methods: In ASCERTAIN, patients receiving background csDMARDs were randomized 1:1:2 to sarilumab 150 mg q2w SC, sarilumab 200 mg q2w SC, or tocilizumab every 4 weeks (q4w) intravenously (IV) starting at 4 mg/kg and increasing to 8 mg/kg if clinically indicated. Patients were eligible to enroll in EXTEND and receive open-label sarilumab 200 mg q2w after completing ASCERTAIN. Clinical endpoints were based on all available data as observed and were summarized through week 84 of EXTEND according to the original randomized treatment. Definitions used as cutoffs for remission and low disease activity (LDA) were DAS28-CRP <2.6 and <3.2 and clinical disease activity index (CDAI) ≤2.8 and ≤10.0, respectively. All patients who had not achieved individual parameters of remission, LDA, and ACR20/50/70 at enrollment into EXTEND were classified as nonresponders for that specific category.
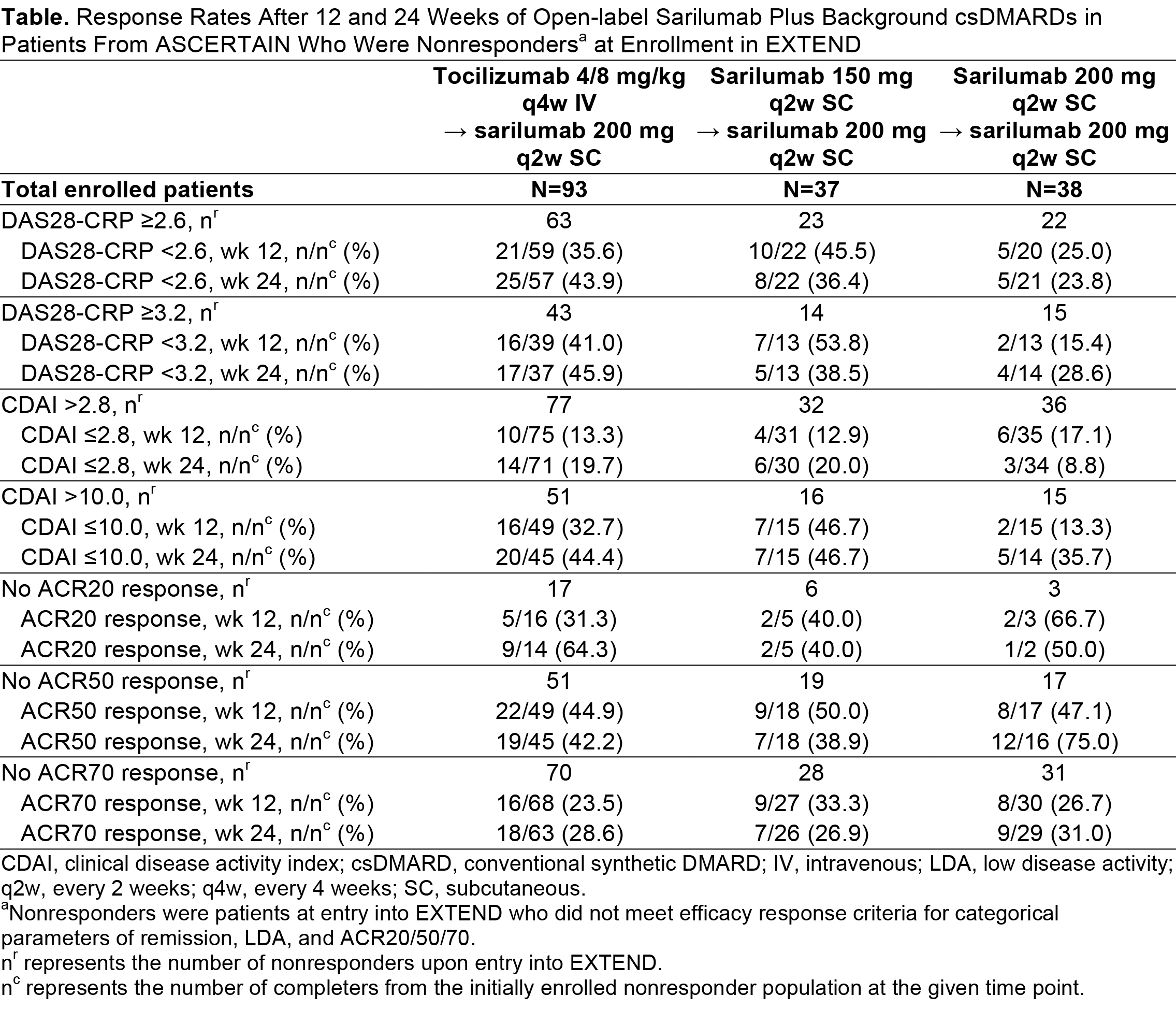
Results: Of the 175 patients who completed ASCERTAIN, 93/96 from the tocilizumab group, 37/40 from the sarilumab 150 mg group, and 38/39 from the sarilumab 200 mg group entered EXTEND. Improvements observed in DAS28-CRP and CDAI in ASCERTAIN were maintained after the switch to open-label sarilumab in EXTEND through week 84 (data not shown). Regardless of initial treatment in ASCERTAIN, the proportions of patients who achieved ACR20/50/70 response, and DAS28-CRP and CDAI remission and LDA increased after switch to sarilumab 200 mg q2w in EXTEND (Table). The greatest increase in patients meeting definitions of remission and LDA was observed in patients initially receiving tocilizumab. Generally, a greater proportion of nonresponders who switched from sarilumab 150 mg q2w to 200 mg q2w achieved ACR20/50/70 response, and DAS28-CRP and CDAI remission and LDA compared with nonresponders who were maintained on 200 mg q2w. The most common treatment-emergent adverse events observed in the EXTEND safety population, which included all patients enrolled from ASCERTAIN and 4 other sarilumab studies, were infections and neutropenia.
Conclusion: Efficacy was maintained or improved in patients rolling over from ASCERTAIN into EXTEND. Increased efficacy was seen in all nonresponder categories, with the largest increases occurring in those who initially received tocilizumab. Although based on a limited population, these findings suggest that switching tocilizumab nonresponders to sarilumab may have favorable efficacy outcomes.
To cite this abstract in AMA style:
Emery P, van Hoogstraten H, Jayawardena S, Mangan EK, Cejas P, Verschueren P. Efficacy of Sarilumab in Patients with Rheumatoid Arthritis Who Previously Received Sarilumab or Tocilizumab [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-sarilumab-in-patients-with-rheumatoid-arthritis-who-previously-received-sarilumab-or-tocilizumab/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-sarilumab-in-patients-with-rheumatoid-arthritis-who-previously-received-sarilumab-or-tocilizumab/