Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Via a systematic literature review of contemporary efficacy data of pharmacological therapies in RA we sought to inform the 2019 update of the EULAR recommendations for the management of RA.
Methods: A systematic literature review to investigate the efficacy of any DMARD (conventional synthetic (cs) DMARD, bDMARD, tsDMARD) or glucocorticoid (GC) therapy in patients with RA. MEDLINE, Embase and the Cochrane Library were searched for articles published between 2016 and March 8th 2019. Open-label studies were acceptable for strategy trials or trials investigating treatment changes
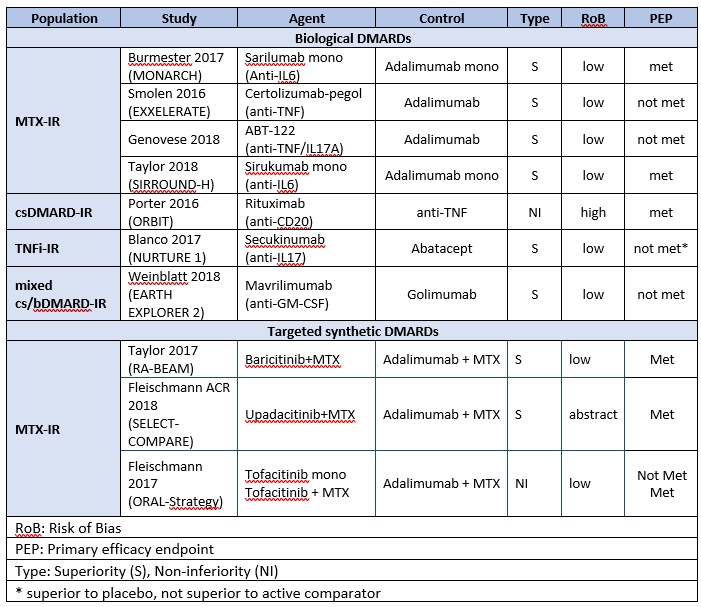
Results: Of 7876 unique abstracts 234 were selected for detailed review with 136 finally included. Twenty-one studies investigated the efficacy of bDMARDs vs. placebo, with 14 studies meeting their primary efficacy endpoint (PEP). Seven head-to-head (H2H) trials comparing bDMARDs and three H2H trials comparing tsDMARD to bDMARDs were included (Table 1). Twenty placebo-controlled trials demonstrated efficacy of JAK inhibitors across different patient populations. Two studies investigated switching of bDMARD therapy in TNFi primary non-responding patients: one trial evaluated the superiority of switching to non-TNFi bDMARDs and met its PEP, while the second evaluated superiority of switching from adalimumab to certolizumab-pegol (CZP) but did not meet its PEP. Stopping or tapering of bDMARDs and/or csDMARDs/GCs was investigated in 22 studies. Using concomitant csDMARDs when tapering bDMARDs lowered the risk of flaring, while tapering csDMARDs in patients with ongoing bDMARD therapy increased the risk of flaring in most studies. All studies investigating biosimilars (n=17) showed non-inferiority compared to their reference products. Further, switching between bDMARD originators and their respective biosimilars showed non-inferiority across all studies (n=11). An open-label strategy trial compared an MRI-guided treat-to-target (T2T) strategy to a conventional T2T strategy (using DAS28-CRP) and failed to show any benefit regarding clinical outcome or radiographic progression. Another open-label strategy RCT stratified patients naïve to csDMARDs according to poor prognostic factors into different csDMARD/GC combination regimes and reported comparable clinical and radiographic results across groups.
Conclusion: The efficacy of many different bDMARDs as well as tsDMARDs was shown in studies included in this SLR. Switching to TNFi or non-TNFi bDMARDs after TNFi treatment failure seems to be feasible. Tapering of bDMARDs as well as csDMARDs is possible in patients achieving long-standing clinical remission but may increase the risk of disease flare. Biosimilars were non-inferior to their reference products.
To cite this abstract in AMA style:
Kerschbaumer A, Sepriano A, Smolen J, van der Heijde D, Dougados M, Van Vollenhoven R, McInnes I, Bijlsma J, Burmester G, de Wit M, Falzon L, Landewé R. Efficacy of Pharmacological Treatment in Rheumatoid Arthritis: A Systematic Literature Review Informing the 2019 Update of the EULAR Recommendations for Management of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-pharmacological-treatment-in-rheumatoid-arthritis-a-systematic-literature-review-informing-the-2019-update-of-the-eular-recommendations-for-management-of-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-pharmacological-treatment-in-rheumatoid-arthritis-a-systematic-literature-review-informing-the-2019-update-of-the-eular-recommendations-for-management-of-rheumatoid-arthritis/