Session Information
Date: Wednesday, November 16, 2016
Title: Systemic Sclerosis, Fibrosing Syndromes, and Raynaud's – Clinical Aspects and Therapeutics III
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Assess the efficacy of mycophenolate mofetil (MMF) and cyclophosphamide (CYC) on the modified Rodnan skin score (mRSS) in patients enrolled in the Scleroderma Lung Study (SLS)-I and II.
Methods: SLS-I participants received daily oral CYC or matching placebo for one year followed for an additional year off therapy, whereas SLS-II participants received daily MMF for 2 years or daily oral CYC for 1 year followed by placebo for a second year. We assessed the within-treatment impact of MMF and CYC on mRSS in subjects with diffuse cutaneous systemic sclerosis (dcSSc) and limited cutaneous systemic sclerosis (lcSSc) participating in SLS-II over a 24-month period. We also compared the change in mRSS in the patients with dcSSc assigned to CYC and MMF in SLS-II and to the CYC group arm of SLS I with the change in the placebo arm of SLS I over a 24-month period.
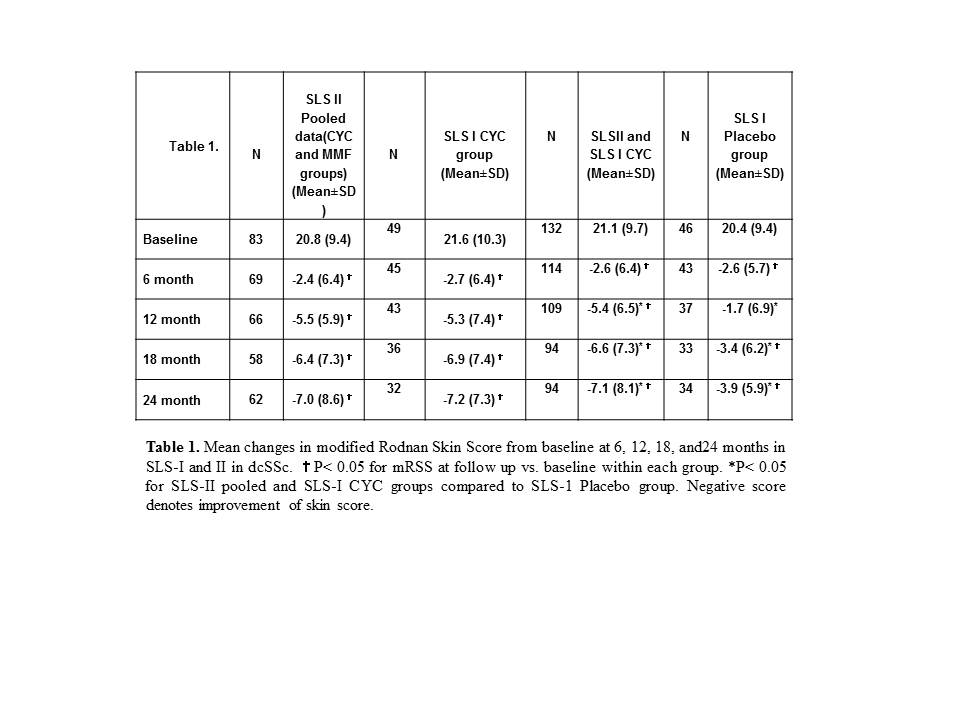
Results: In SLS-II, the baseline (mean± SD) mRSS was 14.0±10.6 for CYC and 15.3±10.4 for MMF; 58.5% were classified as dcSSc. Each treatment was associated with a significant improvement in mRSS from baseline over 24 months (P< 0.05 at each time point, Figure 1. A and B) but there were no significant differences between the2 groups at any time point in either the dcSSc or lcSSc subset (p> 0.05 for all comparisons). In the dcSSc subgroup, the changes in mRSS from baseline to 6-, 12-, 18-, and 24-months were similar in the SLS-II pooled cohort (MMF+ CYC) and the SLS-I CYC cohort and showed statistically significant improvements compared to the SLS-I placebo group at 12, 18, and 24 months (Table 1).
Conclusion: In SLS-II, MMF and CYC each resulted in significant improvement from baseline in mRSS in the subset of patients with dcSSc over a 24-month period. In addition, MMF and CYC each resulted in statistically significant improvements in mRSS over the placebo group in the subjects with dcSSc at 12, 18, and 24 months.
To cite this abstract in AMA style:
Namas R, Tashkin DP, Wilhalme H, Furst DE, Tseng CH, Roth M, Kafaja S, Volkmann ER, Clements PJ, Khanna D. Efficacy of Mycophenolate Mofetil and Oral Cyclophosphamide on Skin Thickness: Post-Hoc Analyses from the Scleroderma Lung Study I and II [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-mycophenolate-mofetil-and-oral-cyclophosphamide-on-skin-thickness-post-hoc-analyses-from-the-scleroderma-lung-study-i-and-ii/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-mycophenolate-mofetil-and-oral-cyclophosphamide-on-skin-thickness-post-hoc-analyses-from-the-scleroderma-lung-study-i-and-ii/