Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Non-infectious uveitis (NIU), inflammation of the eye, can occur in isolation, as in idiopathic uveitis (29% of all pediatric diagnosis), but may also be associated to underlying conditions, such as juvenile idiopathic arthritis-associated uveitis (JIA-U) (21%). As chronic uveitis in children can cause cataracts, glaucoma, and ultimately vision loss, prompt treatment is important.
The first line of treatment for NIU ncludes the use of topical steroids, followed by DMARDs, namely methotrexate, and bDMARDS, primarily TNF inhibitors. Both adalimumab (ADA) and infliximab (IFX) have been shown to effectively reduce uveitis activity and topical steroid requirement.
In case of treatment failure with first anti-TNF therapy (ADA) at weight based standard dosing, the ACR/Arthritis Foundation conditionally recommends escalation of the dose and/or shortening the dosing interval before switching to an alternative anti-TNF or another agent.
There are emerging evidence supporting the efficacy and safety of off-label weekly ADA in refractory NIU in adults and children, including in in JIA. No studies have shown a significant difference between increasing the dosage of ADA instead of shortening the dosing interval. We conducted a retrospective monocentric study on patients affected by refractory/resistant chronic non-infectious uveitis at Children’s Hospital of Philadelphia (CHOP) to compare the effectiveness of an escalated dose of ADA with a shortened timing interval.
Methods: We conducted a retrospective cohort study of children with anterior uveitis from a pediatric tertiary care center between 2013-22. Patients had active uveitis at cohort entry and “control” was the achievement of 2 visits with quiet uveitis (< 1+ AC cell and < 2 drops steroids). Patients could be counted in standard ADA dosing and increased ADA dosing cohort. Standard ADA dosing was weight based q14 days, increased doses was either weekly or higher absolute dose q14 days. We performed standard descriptive statistics and used survival analyses to explore differences in time to achievement of control by ADA use patterns. Analyses were performed using R version 4.4.1.
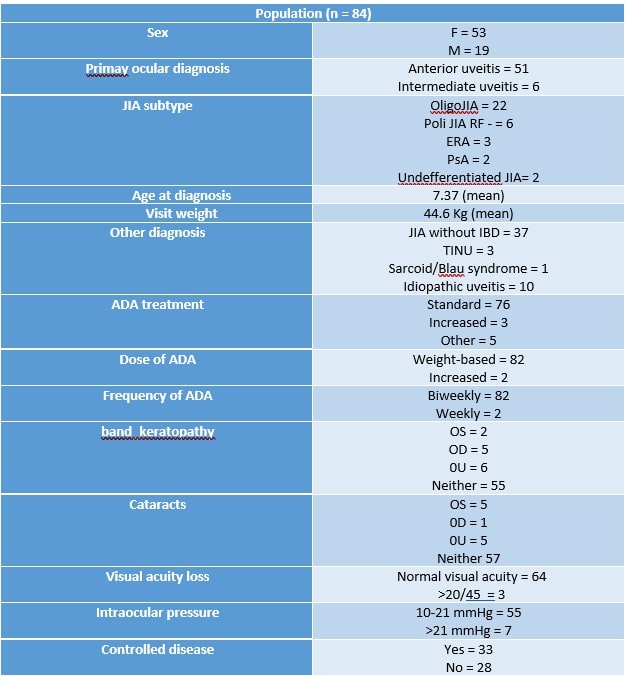
Results: We gathered data from 84 consecutive patients from CHOP affected by any kind of uveitis on treatment with ADA.
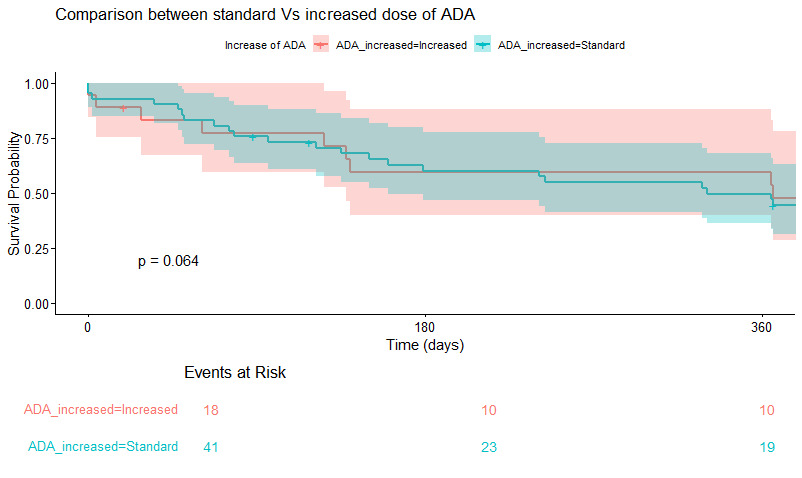
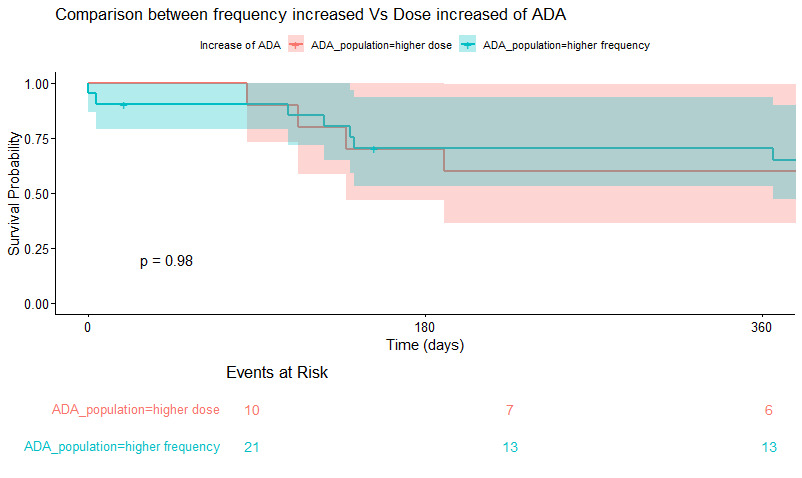
73.6% were female, 89.47% were affected by anterior chronic uveitis while 26.32% by intermediate uveitis (with anterior involvement). 59.4 % were affected by Oligo JIA, 16.2% were PolyJIA RF -, 5% were affected by TINU and 17.5% of the cohort was affected by idiopathic uveitis. Time to control was not significantly faster in episodes of increased ADA vs standard (Figure 1, P=0.06), even when adjusting for disease activity at onset (not shown). Time to control was not impacted by pattern of ADA dose increase (dose vs frequency) (Table 2, p=0.98).
Conclusion: This study explores the optimal method of increase dosing of Adalimumab, although we were unable to show a difference in the outcome between increase dose vs higher frequency. More complex statistical analysis modelling may reveal differences between these groups, enabling us to reach a higher level of care for these patients.
To cite this abstract in AMA style:
Lerman M, Eckardt D, Germinario S. Efficacy of Modified Dosing/interval Timing of Adalimumab in Patients Affected by Chronic Non Infectious Uveitis: A Retrospective Monocentric Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-modified-dosing-interval-timing-of-adalimumab-in-patients-affected-by-chronic-non-infectious-uveitis-a-retrospective-monocentric-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-modified-dosing-interval-timing-of-adalimumab-in-patients-affected-by-chronic-non-infectious-uveitis-a-retrospective-monocentric-study/