Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) are a rare and heterogeneous group of systemic autoimmune disorders, comprising dermatomyositis (DM), immune-mediated necrotizing myopathy (IMNM), polymyositis (PM), inclusion body myositis (IBM) and overlap myositis in which antisynthetase syndrome (ASS) is included. Treatment remains empiric as no on-label drug has been approved so far, with outcomes unsatisfactory in a great number of cases. Most patients accumulate damage with impact in their quality of life, most of it derived directly or indirectly from high cumulative dosages of steroids.
JAK inhibitors (JAKi), targeting IFN pathway, have been used in refractory cases of DM, but effectiveness and safety of their use in other IIM remain to be addressed in clinical trials.
Methods: JAKi were used in a series of 8 patients with refractory IIM, from a single centre, between May 2019 and December 2021. Demographic and clinical data were addressed. Efficacy of treatment was assessed by clinical, laboratory and radiologic findings. Clinical response was defined by 2016 ACR/EULAR Criteria for Minimal, Moderate, and Major Clinical Response in Adult DM and PM as well as the magnitude of steroids weaning. Global tolerance and safety was evaluated.
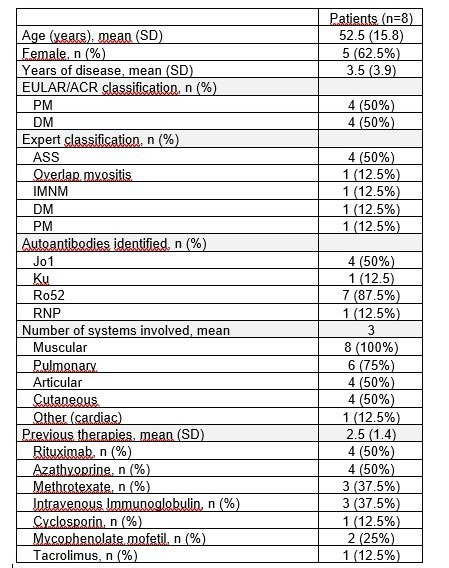
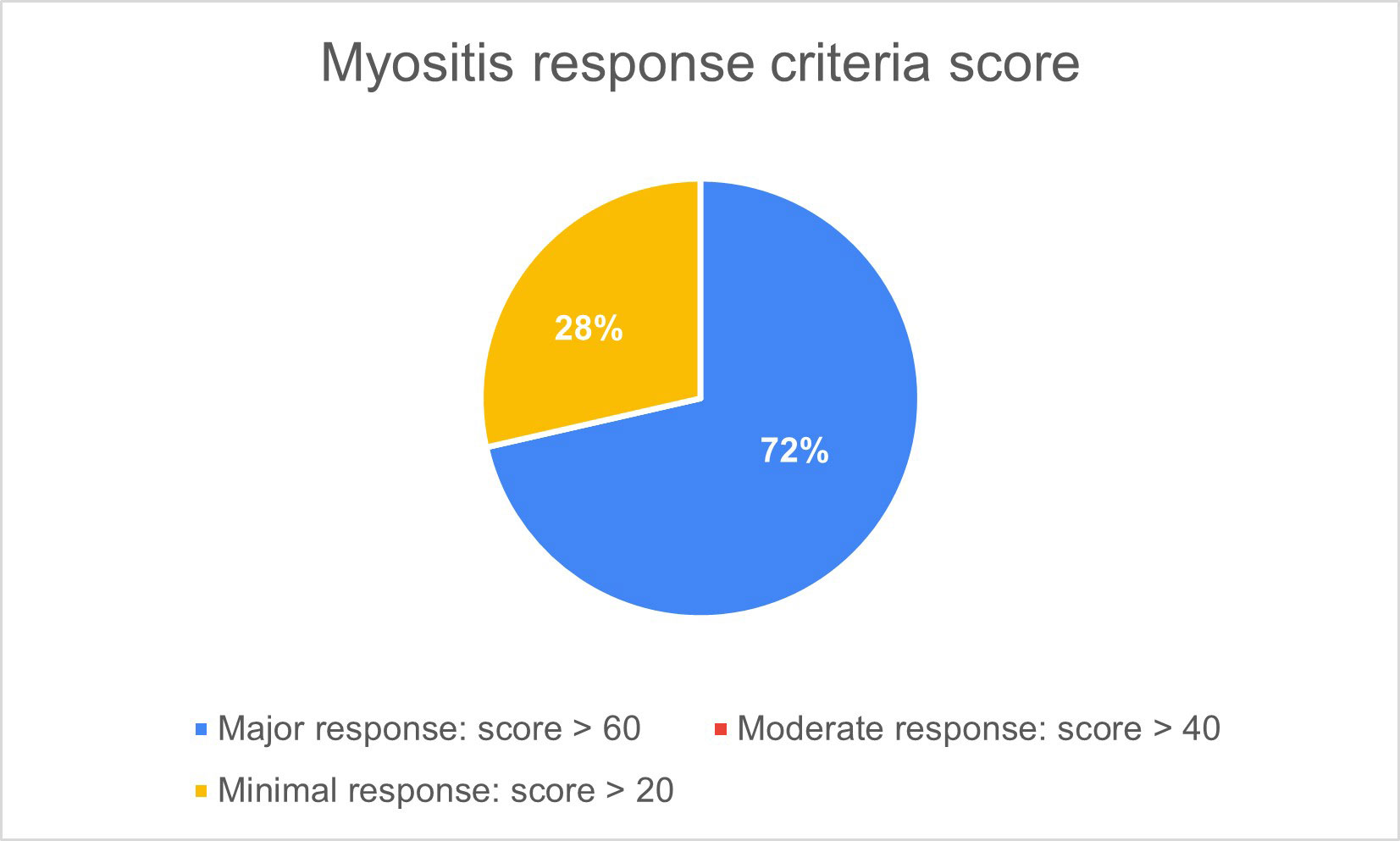
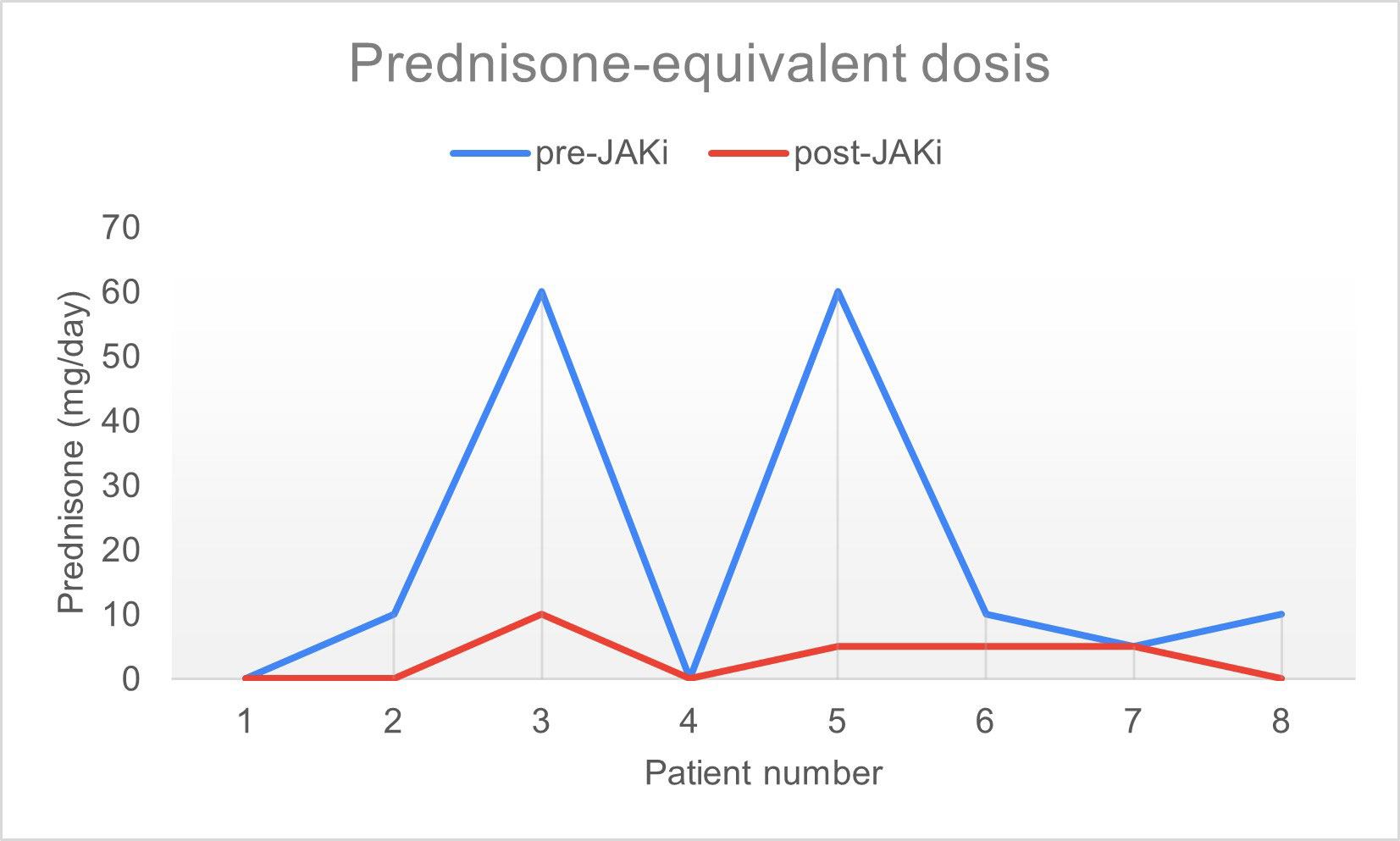
Results: Women represented 62.5% and mean age was 52.5 years old. They all had the diagnosis of IIM for at least 1 year and were refractory to conventional therapies. Half of patients had ASS and 75% had pulmonary involvement (Table 1). Baricitinib was used in 5 patients and tofacitinib in 3 patients. One patient used JAKi as monotherapy and the remaining 7 were under combined therapy with either systemic steroids, intravenous immunoglobulin, rituximab, methotrexate, azathioprine and/or mycophenolate mofetil. Seven patients (87.5%) reporteded improvement of muscular strength, overall health, and quality of life. There was no difference when evaluating JAK inhibitors influence on ESR and CRP. Regarding CPK, a reduction was seen 6 months after initiating the drug in 6 out of 8 patients. Two patients had radiological improvement of pulmonary ground glass infiltrates confirmed by CT-scan. Median score of improvement was 75.4, with 5 out of 8 patients presenting a major response (score > 60), applying the 2016 ACR/EULAR criteria for clinical response (figure 1). Median dosage of systemic steroids significantly decreased after initiation of JAKi, from 19.3mg/day to 3.1mg/day (figure 2). Mean treatment duration settled at 7 months. The drug was discontinued in 2 patients: one, after 10 months, due to absence of response and in the other, after 1 month, because of a serious adverse effect (diverticulitis). In the remaining patients JAKi were well tolerated without serious adverse events.
Conclusion: In this small series of patients, effectiveness was achieved at the muscular level but also in organs not usually targeted in clinical trials of IIM patients, namely lung and articular involvements. JAKi may represent a leap towards more targeted therapies, beyond IFN blockade. It is vital to continue to investigate the exact pathogenic mechanism of the JAK/STAT pathway in IIM and determine the safety and efficacy of the use of multiple JAK-inhibitors as a therapy for treatment-resistant adult IIM.
PM – Polymyositis; DM – Dermatomyositis; IMNM – Immune-mediated necrotizing myopathy; ASS – Anti-synthetase syndrome.
To cite this abstract in AMA style:
Campar A, Sá A, Oliveira B, Marinho A. Efficacy of JAK Inhibitors in Idiopathic Inflammatory Myopathies (other Than Dermatomyositis) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-jak-inhibitors-in-idiopathic-inflammatory-myopathies-other-than-dermatomyositis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-jak-inhibitors-in-idiopathic-inflammatory-myopathies-other-than-dermatomyositis/