Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Despite the use of corticosteroids in acute gout, there exist wide variations in treatment doses and duration. No studies have evaluated the ideal dose of steroids for gout flares. However, occurrences of treatment failure and side effects from treatment warrant an evaluation for the lowest effective dosing. The goal of this double blinded prospective pilot study is to compare efficacies of a moderate dose and high dose prednisone course in treating acute gout.
Methods:
Male subjects age 18 years and older at Edward Hines Jr Hospital VA with acute gout were included. Patients with heart failure exacerbation, uncontrolled diabetes mellitus, infection, pseudogout, or chronic prednisone use were excluded. Subjects were randomized to a high or moderate dose of prednisone (high: 60mg on day 1, decreased by 10mg each day for a 6 day course; moderate: 30mg on day 1, decreased by 5mg each day for 6 days).
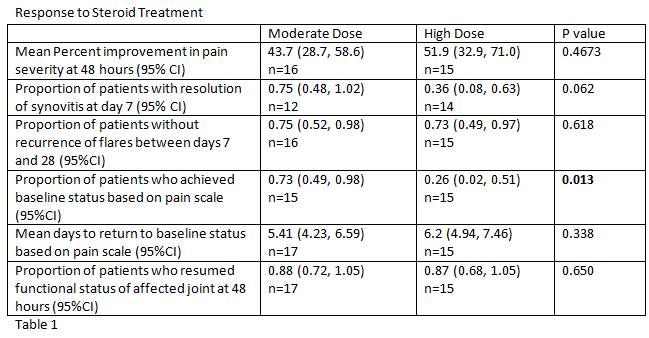
The primary endpoint was improvement in pain at 48 hours based on a 200mm visual analog scale. Secondary endpoints included the proportion of patients with resolution of synovitis by Day 7; absence of recurrent flares at 28 days; the proportion of patients who returned to baseline pain by day 7; and the number of days to return to baseline pain levels.
An intention-to-treat approach was used for analysis. Student t-test was used to compare mean values and Fishers exact test was used to compare proportions (STATA 11.1).
Results:
139 patients were screened, and 33 patients were enrolled between September 2007 and December 2014. All patients enrolled were male, with a mean age of 62.2 (± 11.8) and 63.4 (± 15.8) years in the moderate and high dose groups respectively. Results are summarized in Table 1. The mean percent improvement in pain severity at 48 hours was higher in the high dose group (51.9 vs 43.7%), though it did not achieve statistical significance. Of 26 patients examined at day 7, a higher proportion of patients in the moderate dose group experienced resolution of synovitis, though this finding did not reach statistical significance (0.75 in moderate group vs 0.36 in high group).
The proportion of patients back at baseline pain on day 7 was significantly higher in the moderate dose group (0.73 vs 0.26, p=0.013). The time to return to baseline pain levels was also shorter in the moderate dose group but did not achieve statistical significance.
Conclusion:
In this randomized double blinded pilot study, there were no significant differences between the response of acute gout to high dose versus moderate dose prednisone at 48 hours and exam findings on day 7. Furthermore, the proportion of patients who returned to baseline pain levels was lower in the high dose group, contrary to what investigators hypothesized would occur. Despite size limitations of this pilot study, our findings warrant larger trials to establish a standardized, evidence based practice for appropriate dosing of steroids in acute gout.
To cite this abstract in AMA style:
Ostrowski RA, Araujo E, Hariman R, Adams E. Efficacy of High Dose Versus Moderate Dose Prednisone in the Treatment of Acute Gout [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-high-dose-versus-moderate-dose-prednisone-in-the-treatment-of-acute-gout/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-high-dose-versus-moderate-dose-prednisone-in-the-treatment-of-acute-gout/