Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Late-onset PsA may be associated with elevated acute phase reactants, higher joint count, and erosive disease at diagnosis1,2. Given potential phenotype differences and increasing prevalence with aging populations, it is important to establish the efficacy of current treatments in late-onset PsA. Previous analyses of DISCOVER-1&2 (D1&2) have shown the fully human IL-23p19-subunit inhibitor guselkumab (GUS) to be associated with robust and sustained improvement in PsA signs/symptoms over 52 weeks (W) in subgroups of patients (pts) across a variety of baseline (BL) characteristics3. These post hoc analyses contrasted early- vs. late-onset PsA pts and evaluated GUS efficacy through W52 in both cohorts.
Methods: Adults in D1&2 (90% bionaive) with active PsA despite standard therapies were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo (PBO) with crossover to GUS 100 mg Q4W at W24. The tertile (T) distribution of age at PsA diagnosis in the pooled D1&D2 population informed the definition of early- (T1 < 36y) and late- (T3 ≥47y) onset. W24/52 efficacy assessments included least-square mean changes in Disease Activity Index for PsA (DAPSA), swollen/tender joint counts (SJC/TJC), Psoriasis Area Severity Index (PASI; in pts with BSA≥3% and IGA≥2 at BL), CRP, pt-reported pain (Pt-Pain), pt global assessment of arthritis and psoriasis (PtGA), Short Form 36-item physical component summary score (SF-36 PCS), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI; in pts with axial involvement [axPsA]), and Ankylosing Spondylitis Disease Activity Score (ASDAS; in pts with axPsA), as well as ACR and Minimal Disease Activity (MDA) response rates (employing non-responder imputation [NRI] for missing data).
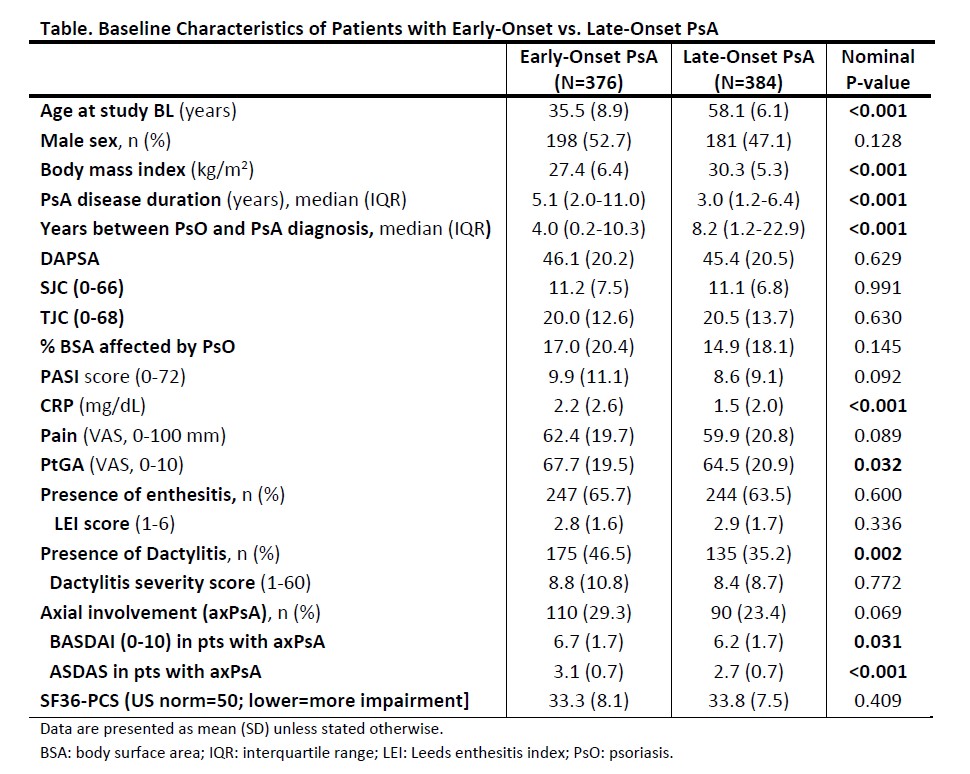
Results: Relative to pts with late-onset (n=384), those with early-onset (n=376) PsA were significantly younger at BL but had longer PsA duration and a shorter interval between psoriasis and PsA diagnoses (Table 1). The two cohorts had generally comparable joint and skin disease severity at BL, although those with early-onset PsA were more likely to have axPsA and dactylitis, and exhibited higher CRP levels, PtGA, BASDAI and ASDAS.
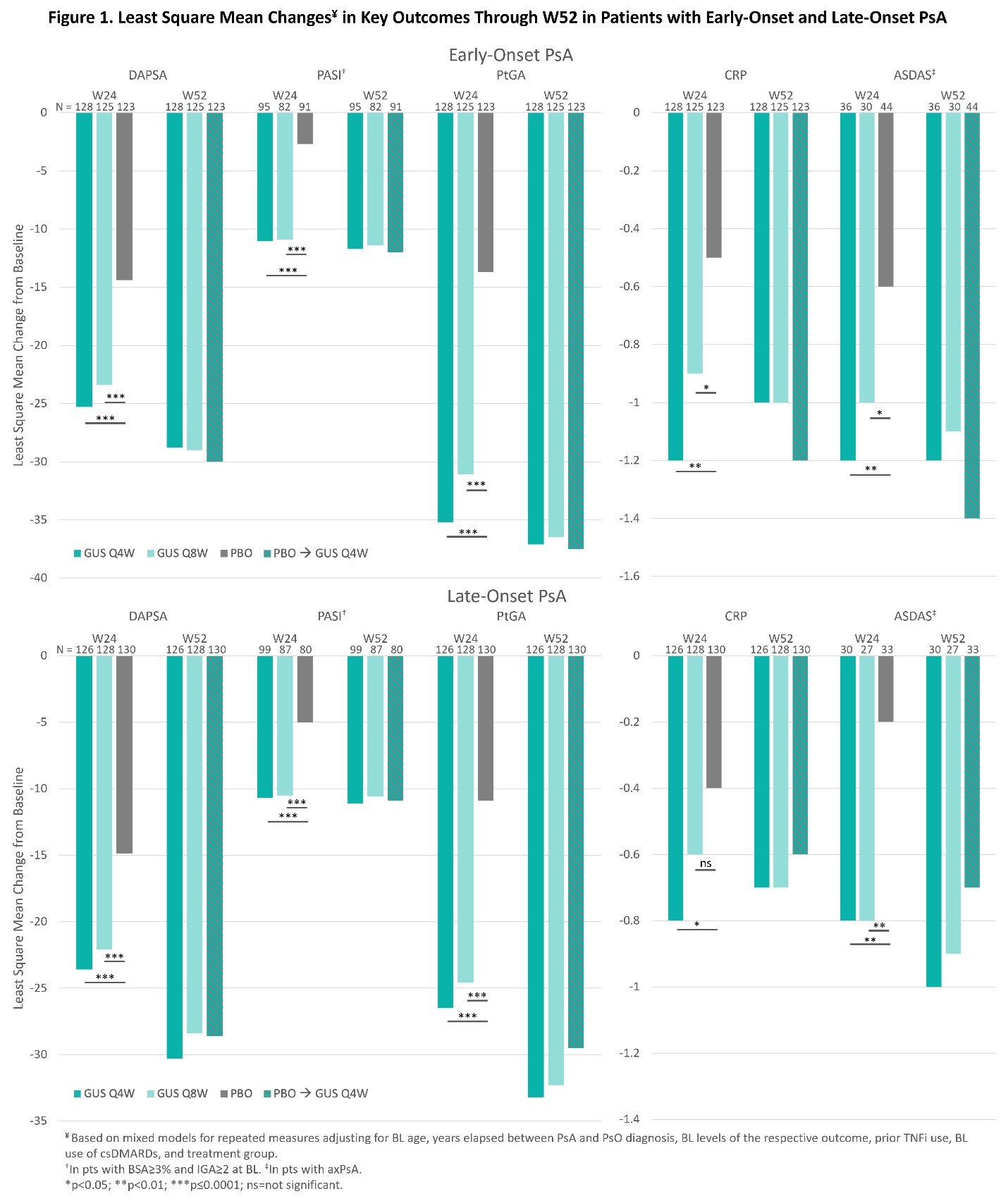
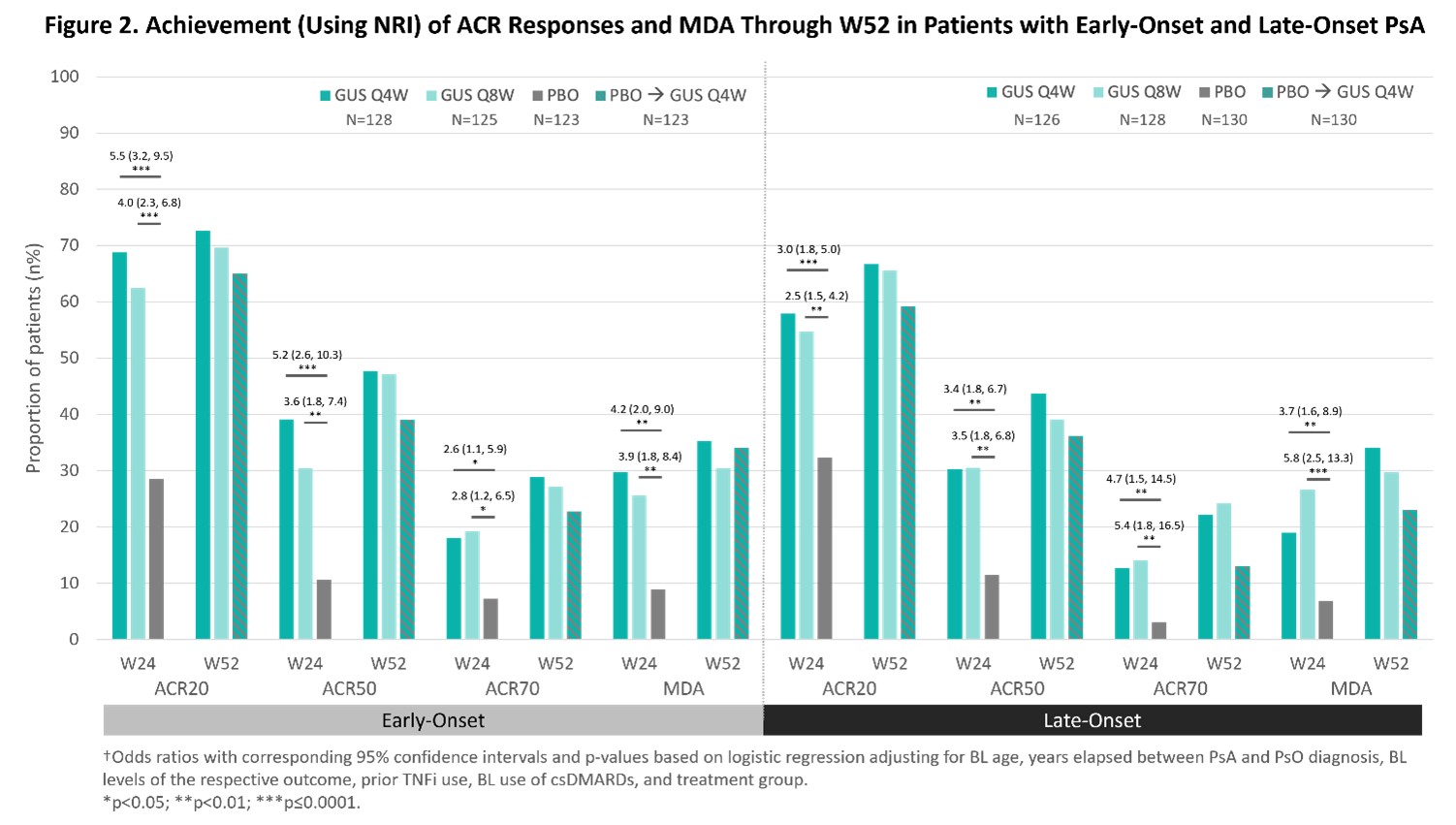
Irrespective of PsA onset age, GUS Q4W or Q8W was associated with significantly (nominal p< 0.001) greater improvements in DAPSA, PASI, PtGA, CRP, ASDAS (Figure 1), SJC, TJC, Pt-Pain, BASDAI, and SF-36 PCS (data not shown), and higher ACR20/50/70 and MDA response rates vs. PBO at W24 (Figure 2). Further improvements/increased response rates were generally seen through W52 of GUS across early- and late-onset subgroups (Figure 1, 2).
Conclusion: Results of post hoc analyses, conducted in a large cohort of mainly bio-naïve pts with active PsA from D1&2, suggest early-onset PsA may be associated with more aggressive presentation several years post-diagnosis. Irrespective of age of PsA onset and differences in baseline profile, however, GUS was associated with significant and clinically meaningful improvements across key PsA domains, with further enhancements generally seen through W52.
References:
1. Fragoulis GE. J Rheumatol 2022;49:1085
2. Polachek A. Semin Arthritis Rheum 2019;48:834
3. Ritchlin CT. RMD Open 2022;8:e002195
To cite this abstract in AMA style:
Soriano E, Kishimoto M, Rampakakis E, Nantel F, Shawi M, Lavie F, Mease P, Gladman D. Efficacy of Guselkumab in Early-Onset and Late-Onset Psoriatic Arthritis: Post Hoc Pooled Analyses of Two Phase 3 Randomized Controlled Trials in Patients with Active PsA [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-guselkumab-in-early-onset-and-late-onset-psoriatic-arthritis-post-hoc-pooled-analyses-of-two-phase-3-randomized-controlled-trials-in-patients-with-active-psa/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-guselkumab-in-early-onset-and-late-onset-psoriatic-arthritis-post-hoc-pooled-analyses-of-two-phase-3-randomized-controlled-trials-in-patients-with-active-psa/