Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In the treatment of RA, JAK inhibitors are a valuable option to meet remission or low disease activity (LDA) treatment targets following an inadequate response (IR) or intolerance to ≥ 1 conventional synthetic disease-modifying antirheumatic drug (DMARD). Filgotinib (FIL) is a JAK1 preferential inhibitor available in two doses for the treatment of moderate to severe RA. The objective of this analysis was to evaluate long-term efficacy of two doses of FIL in clinically relevant pt populations. Response rates for Boolean remission 2.0 were reported as an exploratory objective.
Methods: In this interim analysis, efficacy (nonresponder imputation) of FIL 200 mg (FIL200) and 100 mg (FIL100) was assessed from long-term extension (LTE) baseline (BL) to Week (W) 156 in patients (pts) with an IR to methotrexate (MTX-IR) and biologics (bDMARD-IR), enrolled from FINCH 1 (NCT02889796) and 2 (NCT02873936) parent studies, respectively, receiving ≥ 1 FIL dose in FINCH 4 (NCT03025308).
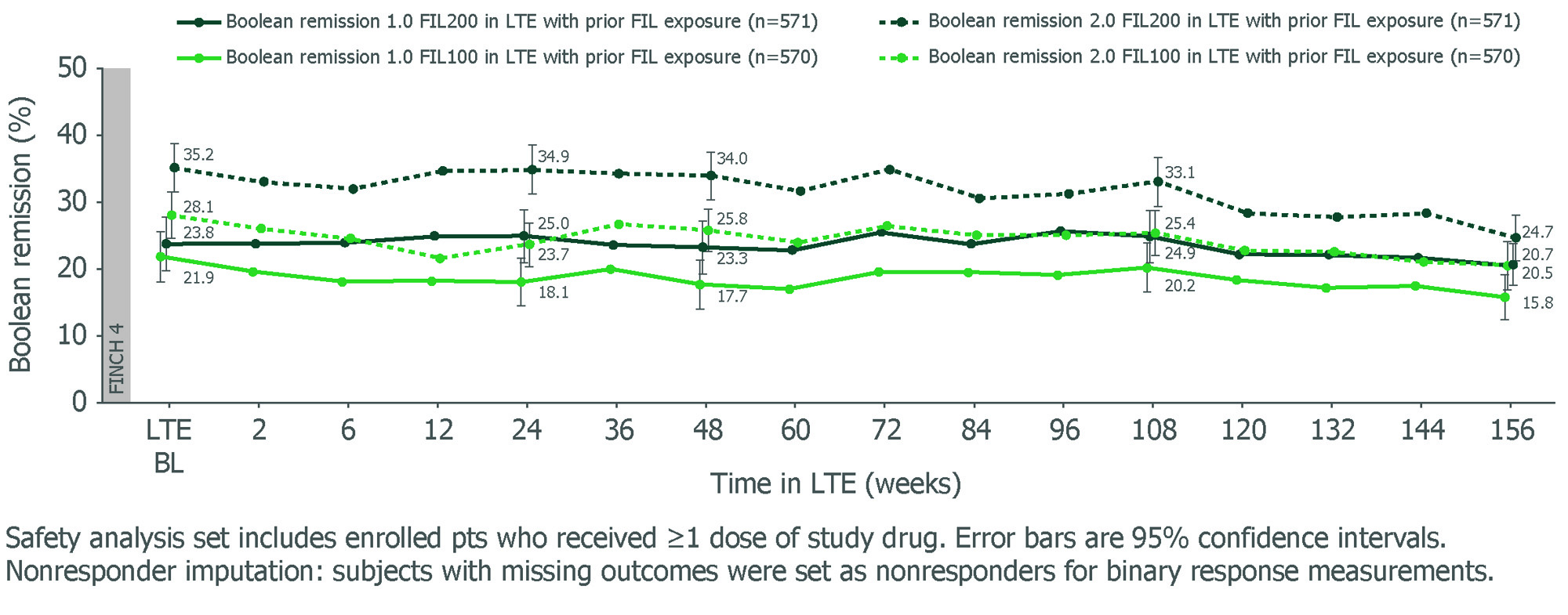
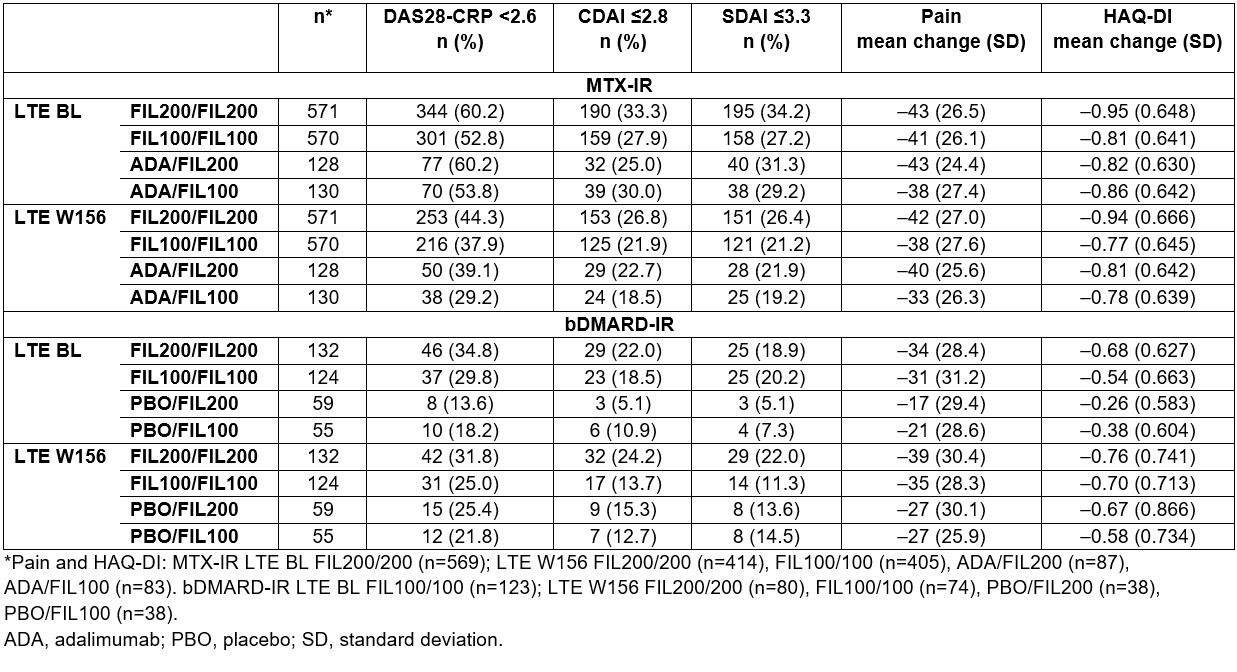
Results: Study design, BL characteristics and W48 outcomes for MTX-IR1 and bDMARD-IR2 pts were reported previously. For MTX-IR and bDMARD-IR pts who received FIL200 or FIL100 in the parent study, W156 remission rates using Boolean 1.0 criteria were 20.5% and 15.8%, and 18.2% and 8.9%, respectively. Adopting the Boolean 2.0 criteria slightly increased remission rates for FIL200 and FIL100 at W156: +4.2% and +4.9% for MTX-IR pts, and +1.5% and +2.4% for bDMARD-IR pts, respectively. For pts rerandomized to FIL on entering the LTE, Boolean 2.0 criteria also increased remission rates vs Boolean 1.0. Both MTX-IR (Figure) and bDMARD-IR pts maintained long-term Boolean remission through W156 with FIL200 and FIL100, irrespective of prior FIL. Results for Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) < 2.6, Clinical Disease Activity Index (CDAI) ≤ 2.8 and Simplified Disease Activity Index (SDAI) ≤ 3.3, and mean change from parent study BL in Health Assessment Questionnaire–Disability Index (HAQ-DI) and pain are shown (Table). Similar trends in efficacy were seen for LDA and ACR response criteria.
Conclusion: In FINCH 4, both FIL200 and FIL100 showed sustained efficacy up to W156 in clinically relevant pt populations. Boolean 2.0 criteria classified more pts in remission, in line with the range reported in the validation study.3
References:
1. Combe B, et al. Arthritis Rheumatol 2021;73(Suppl 9):POS1697
2. Buch M, et al. Arthritis Rheumatol 2021;73(Suppl 9):POS1696
3. Studenic P, et al. Arthritis Rheumatol 2023;82:74–80
To cite this abstract in AMA style:
Buch M, Aletaha D, Caporali R, Combe B, Schulze-Koops H, Gottenberg J, Tanaka Y, Blanco R, Takeuchi T, Ekoka Omoruyi E, Van Beneden K, Rajendran V, Watson C, De Leonardis F, Emery P. Efficacy of Filgotinib in Patients with Rheumatoid Arthritis: Week 156 Results from a Long-term Extension Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-filgotinib-in-patients-with-rheumatoid-arthritis-week-156-results-from-a-long-term-extension-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-filgotinib-in-patients-with-rheumatoid-arthritis-week-156-results-from-a-long-term-extension-study/