Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare inflammatory disorder characterized by small- to medium-vessel vasculitis, asthma and eosinophilia. In the head-to-head MANDARA trial (NCT04157348), patients with EGPA treated with benralizumab achieved non-inferior outcomes, in terms of remission, (defined as Birmingham Vasculitis Activity Score [BVAS] = 0 and oral glucocorticoid [OGC] dose ≤4 mg/day at both Weeks 36 and 48) compared with patients who received mepolizumab. This analysis focuses on changes in individual items of disease activity and damage during the 52-week double-blind phase of the trial.
Methods: 140 adults with relapsing or refractory EGPA receiving standard of care (stable OGCs ± stable immunosuppressive therapy for ≥4 weeks before enrollment) were randomized to benralizumab 30 mg (n=70) or mepolizumab 300 mg (n=70) SC Q4W for 52 weeks. Findings for the BVAS and the Vasculitis Damage Index (VDI) assessments are reported for the combined total trial population.
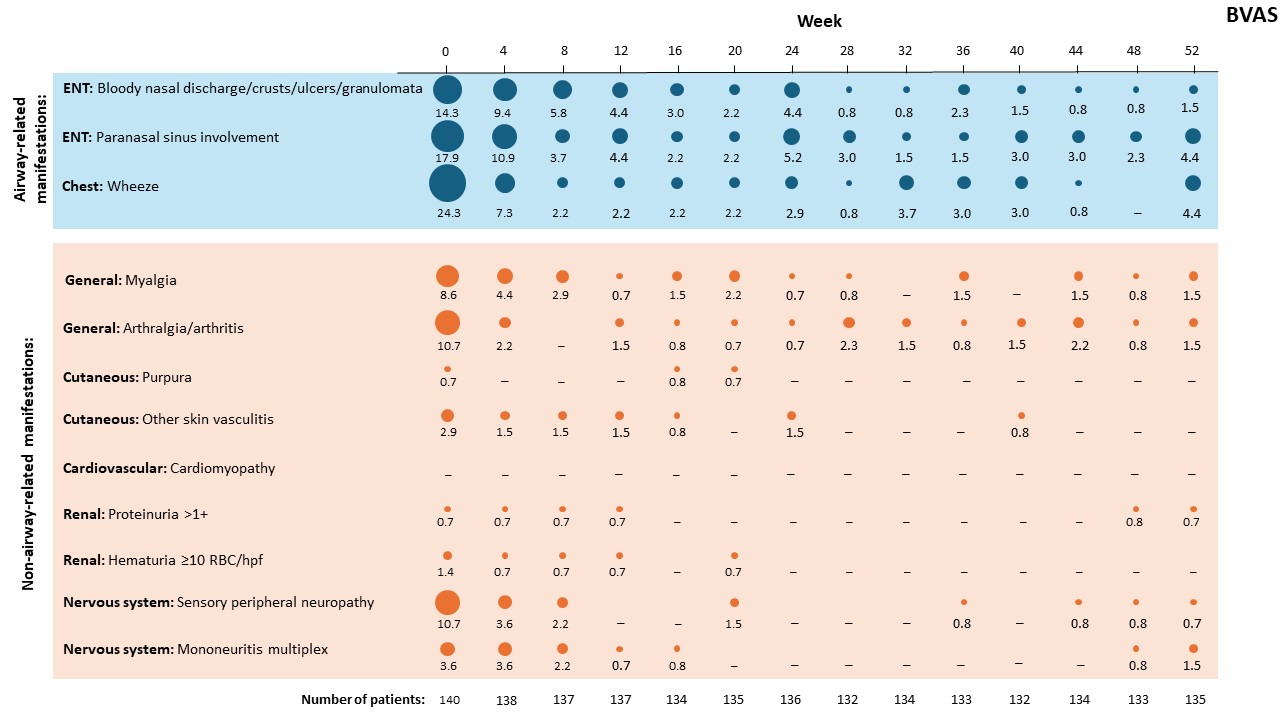
Results: Among the 140 patients in the trial, airway-related manifestations commonly reported at baseline included wheeze (24.3% of patients), paranasal sinus involvement (17.9%), and bloody nasal discharge/crusts/ulcers/granulomata (14.3%), all of which decreased rapidly in frequency and affected < 5% of patients by Week 52, despite substantial OGC reductions during the study (Figure 1). The frequency of non-airway-related manifestations (including manifestations such as arthralgia/arthritis, sensory peripheral neuropathy, myalgia, and mononeuritis multiplex) also decreased to < 2% of patients by Week 52. Cutaneous manifestations were infrequent at baseline, and disappeared by Week 52. No cardiovascular manifestations were reported at baseline or during the trial. Three central nervous system manifestations (sensorineural hearing loss) were reported at baseline but not subsequently during the trial.
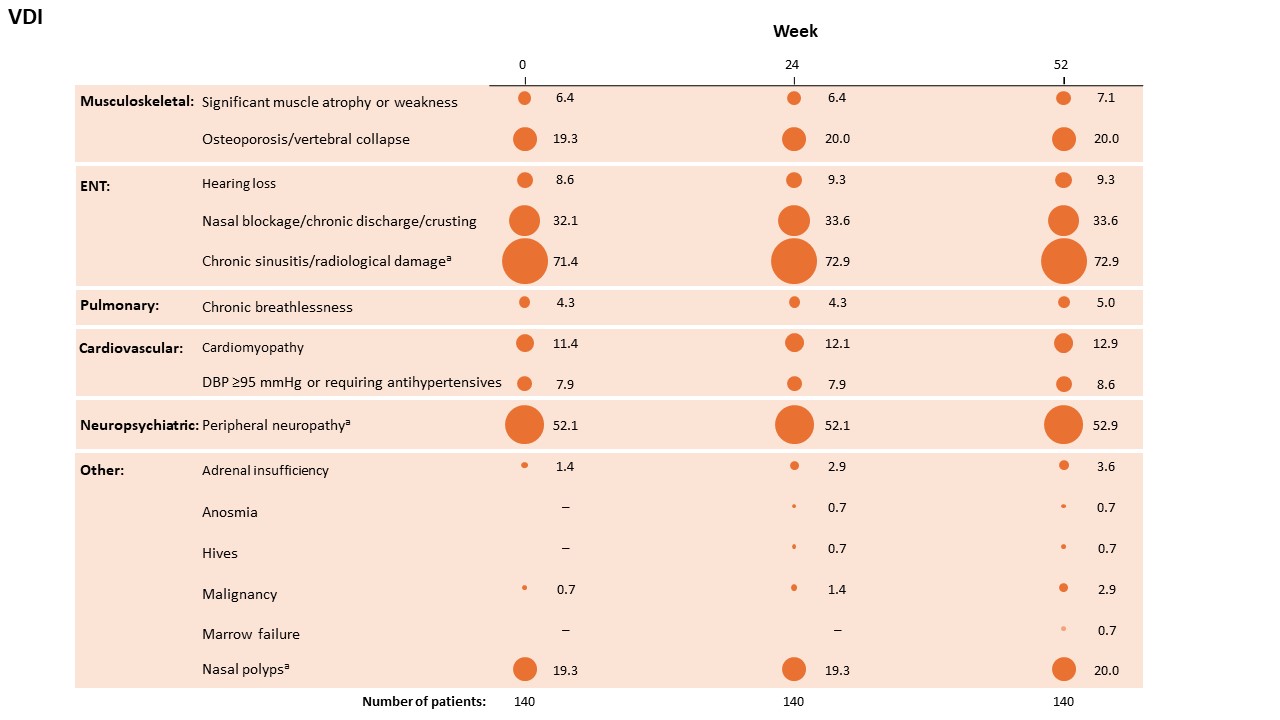
There were 22 new items of damage recorded during the double-blind period (Figure 2). Of these, only 4 (benralizumab, n=2; mepolizumab, n=2) were increases related to persistent disease activity: chronic sinusitis/radiotherapy damage, n=2; peripheral neuropathy, n=1; nasal polyps, n=1. None of these were new manifestations for the patients.
Conclusion: Eosinophil-targeting biologic agents appear to be rapidly effective at reducing and controlling both airway- and non-airway-related manifestations of EGPA. In the 52-week double-blind period of the MANDARA trial, few patients experienced worsening of their disease that progressed to organ damage, suggesting that these therapies may be effective both on airway-related and non-airway-related manifestations of EGPA, despite substantial OGC reductions.
Corresponding proportion of patients (%) is shown beneath each bubble. Manifestations are categorized as per the Birmingham Vasculitis Activity Score.
ENT, ear, nose, throat; hpf, high power field; RBC, red blood cells.
Corresponding proportion of patients (%) is shown beneath each bubble.
a EGPA-related items of the Birmingham Vasculitis Activity Score that progressed to the VDI: ENT (chronic sinusitis/radiological damage; n=2, one from bloody nasal discharge/crusts/ulcers/granulomata and one from paranasal sinus involvement); neuropsychiatric (peripheral neuropathy; n=1 from mononeuritis multiplex); and other (nasal polyps; n=1 from bloody nasal discharge/crusts/ulcers/granulomata and paranasal sinus involvement).
DBP, diastolic blood pressure; ENT, ear nose and throat.
To cite this abstract in AMA style:
Merkel P, Jayne D, Specks U, Pagnoux C, Terrier B, Hellmich B, Necander S, Shavit A, Walton C, Wechsler M. Efficacy of Eosinophil-Targeting Therapies on Specific Disease Manifestations of Eosinophilic Granulomatosis with Polyangiitis in the Phase 3 MANDARA Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-eosinophil-targeting-therapies-on-specific-disease-manifestations-of-eosinophilic-granulomatosis-with-polyangiitis-in-the-phase-3-mandara-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-eosinophil-targeting-therapies-on-specific-disease-manifestations-of-eosinophilic-granulomatosis-with-polyangiitis-in-the-phase-3-mandara-trial/