Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare inflammatory disorder characterized by asthma, eosinophilia, and small-to-medium size vessel vasculitis, with individual manifestations widely ranging in severity. In the Phase 3 MANDARA trial in patients with EGPA (NCT04157348), benralizumab was non-inferior to mepolizumab in inducing remission. This post-hoc analysis examined the efficacy of eosinophil-targeting therapies (benralizumab or mepolizumab) based on severity of patients’ EGPA-associated cumulative manifestations >3 months prior to study enrollment.
Methods: 140 adults with relapsing and/or refractory EGPA treated per standard of care were randomized to benralizumab 30 mg (n=70) or mepolizumab 300 mg (n=70) SC Q4W for 52 weeks. Primary endpoint was the proportion of patients achieving remission (Birmingham Vasculitis Activity Score [BVAS] = 0 and oral glucocorticoid [OGC] dose ≤4 mg/day) at both Weeks 36 and 48. Secondary endpoints were OGC use, clinical benefits, and relapse. In this analysis, data from both treatment groups were pooled to evaluate these endpoints according to presence/absence of severe EGPA, defined as presence ever of at least one of the following: cardiomyopathy, glomerulonephritis, alveolar hemorrhage; or treatment with cyclophosphamide or rituximab. Patients with historic evidence of severe EGPA are referred to as the ‘severe EGPA group’. No patients had a severe manifestation of EGPA at trial entry.
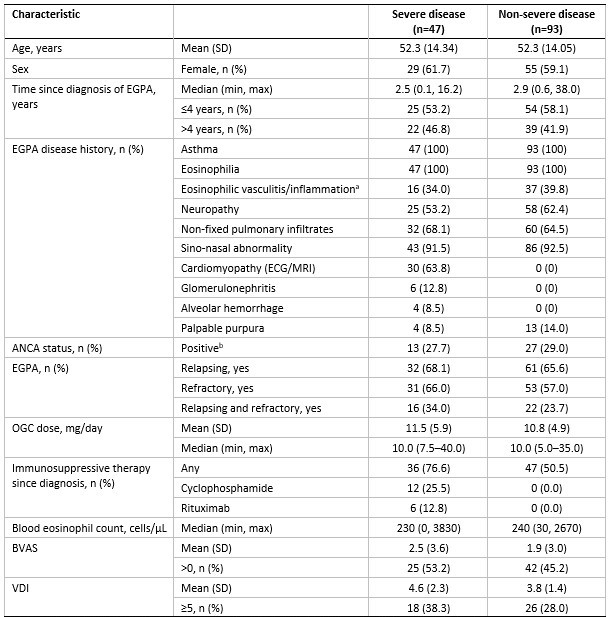
Results: Of 140 randomized patients, 47 (33.6%) had severe EGPA (22 mepolizumab, 25 benralizumab) and 93 (66.4%) had non-severe EGPA (48 mepolizumab, 45 benralizumab). Baseline BVAS and VDI were higher in the severe versus non-severe EGPA group (Table). A higher proportion of patients with severe EGPA had both relapsing and refractory disease and received non-OGC immunosuppressive therapy since diagnosis.
The adjusted rates of remission at both Weeks 36 and 48 were similar regardless of disease severity (severe EGPA: 53.6%, non-severe EGPA: 60.0%; difference: –6.48 [95% confidence interval (CI):
–22.91, 9.95], p=0.4392) as were the adjusted rates for achieving remission by Week 24 and remaining in remission until Week 52 (severe EGPA: 37.9%, non-severe EGPA: 39.9%; difference:
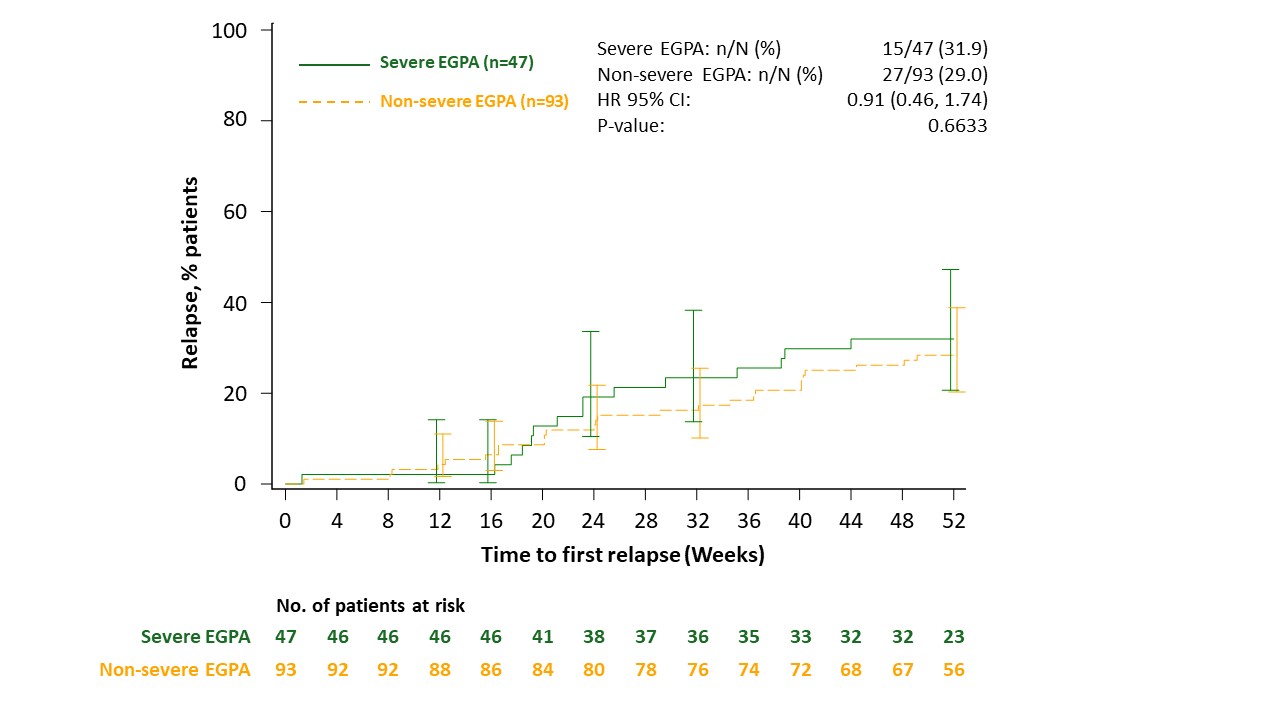
–1.98 [95% CI: –17.40, 13.44], p=0.8014). The proportion of patients with a relapse was also similar between the severe (31.9%) and non-severe (29.0%) groups (Figure). During Weeks 48 through 52, 83.0% and 78.5% of patients in the severe and non-severe EGPA groups, respectively, had a ≥50% reduction from baseline in OGC dose (difference: 4.44 [95% CI: –8.91, 17.79], p=0.5147) and 31.1% versus 34.8%, respectively, were fully tapered off OGCs (difference: –3.64 [95% CI: –19.50, 12.22), p=0.6527).
Conclusion: Eosinophil-targeting therapies (benralizumab or mepolizumab) demonstrated similar efficacy in patients with EGPA with or without a history of severe disease, with results among patients in the former group comparable to the overall cohort in the trial. The data suggest that targeting eosinophils may be effective regardless of history of severe disease in patients with relapsing or refractory EGPA.
Severe EGPA was defined by presence ever of at least one of the following characteristics: cardiomyopathy, glomerulonephritis, alveolar hemorrhage; or treatment with cyclophosphamide or rituximab.
a By biopsy evidence; b Historical or at baseline.
ACQ-6, 6-item Asthma Control Questionnaire; ANCA, anti-neutrophil cytoplasmic antibodies; BVAS, Birmingham Vasculitis Activity Score; ECG, electrocardiogram; EGPA, eosinophilic granulomatosis with polyangiitis; MRI, magnetic resonance imaging; OGC, oral glucocorticoids; SD, standard deviation; SNOT_22, Sino-Nasal Outcomes Test 22; VDI, Vasculitis Damage Index.
Severe EGPA was defined by presence ever of at least one of the following characteristics: cardiomyopathy, glomerulonephritis, alveolar hemorrhage; or treatment with cyclophosphamide or rituximab.
Relapse was defined as worsening or persistence of active disease characterized by: active vasculitis (BVAS >0); or active asthma symptoms and/or signs with a corresponding worsening in ACQ-6 score; or active nasal and/or sinus disease, with a corresponding worsening in at least one of the sino-nasal symptom questions; warranting: an increase of OGC therapy; or an increased dose or addition of an immunosuppressive agent or hospitalization related to EGPA worsening.
ACQ-6, 6-item Asthma Control Questionnaire; BVAS, Birmingham Vasculitis Activity Score; EGPA, eosinophilic granulomatosis with polyangiitis; HR, hazard ratio; OGC, oral glucocorticoid.
To cite this abstract in AMA style:
Hellmich B, Merkel P, Jayne D, Terrier B, Roufosse F, Nair P, Khalidi N, Jackson D, Furuta S, Börjesson Sjö L, Necander S, Shavit A, Walton C, Wechsler M. Efficacy of Eosinophil-Targeting Therapies According to Disease Severity in Patients with Eosinophilic Granulomatosis with Polyangiitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-eosinophil-targeting-therapies-according-to-disease-severity-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-eosinophil-targeting-therapies-according-to-disease-severity-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis/