Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: To date there are no head-to-head trials comparing the efficacy of biologic treatments for polyarticular-course JIA (pcJIA). The purpose of this study was to use statistical methods to estimate the relative efficacy of biologic treatments, alone and in combination with methotrexate (MTX), in the management of pcJIA by means of indirect comparison of randomised controlled trials (RCTs).
Methods: Based on a literature review, we identified RCTs of abatacept,1 adalimumab2 (ADA), etanercept,3 infliximab,4 and tocilizumab5 (TCZ; CHERISH) in pcJIA. Comparative effectiveness was estimated on the reported American College of Rheumatology response rates (JIA ACR30/50/70/90) measured at the end of the randomized, double-blind phase by means of a Bayesian indirect comparison using a fixed-effects ordered probit model. Probabilities of achieving different levels of JIA ACR response were calculated for biologic treatments and placebo using all observed comparisons.
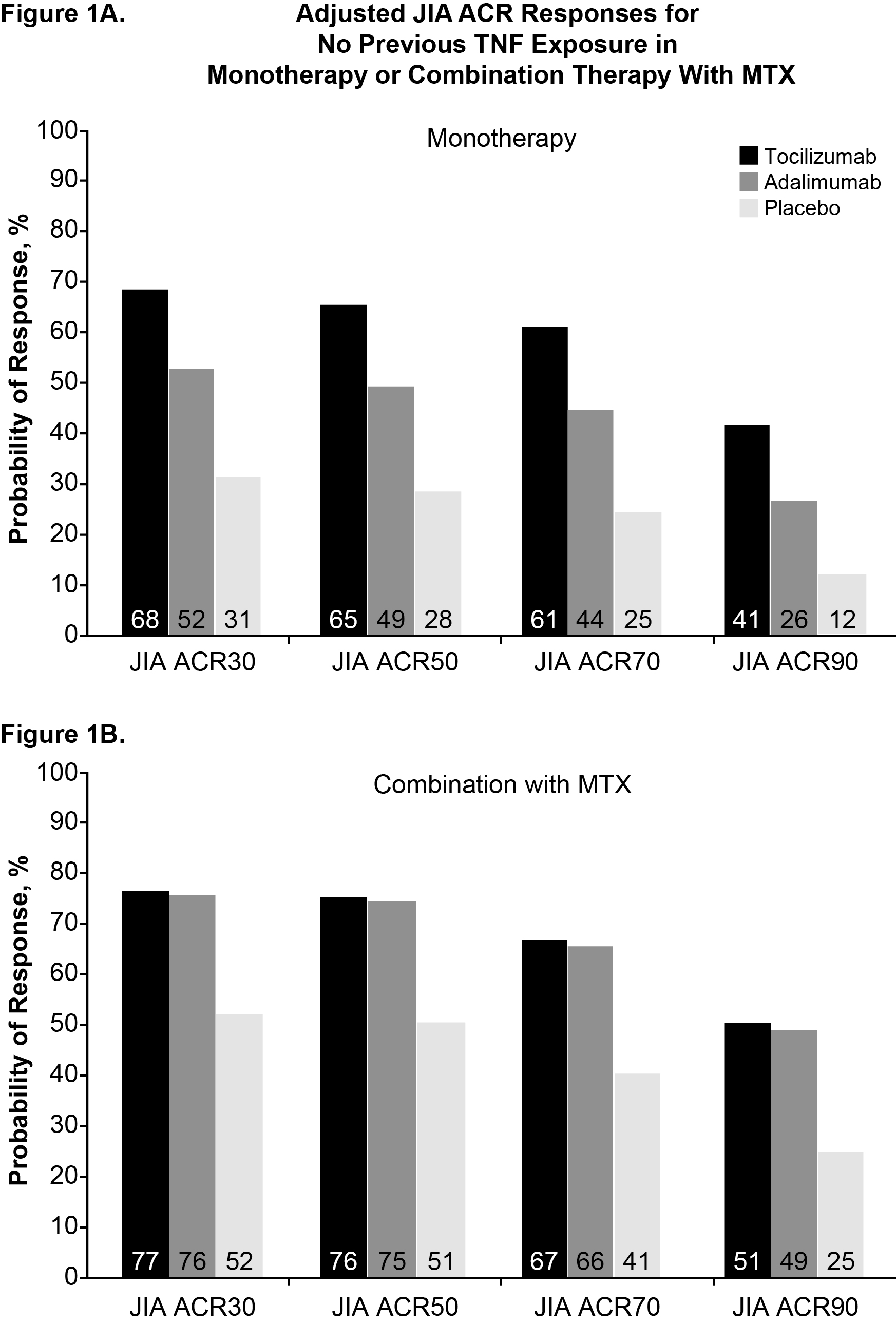
Results: The 5 RCTs identified showed differences in reporting JIA ACR responses with regard to methods of nonresponder imputation during the blinded, controlled phase, allowing only for the comparison of ADA and TCZ. After correction for previous biologic use (Figure), for a JIA ACR30 placebo response of 31%, TCZ monotherapy had a higher predicted probability of achieving JIA ACR30 (68%), JIA ACR50 (65%), JIA ACR70 (61%), and JIA ACR90 (41%) vs ADA monotherapy, with 52%, 49%, 44%, and 26%, respectively. On MTX background therapy and a JIA ACR30 placebo response of 52%, TCZ had a higher expected probability of response at JIA ACR30 (77%), JIA ACR50 (76%), JIA ACR70 (67%), and JIA ACR90 (51%) vs ADA, with 76%, 75%, 66%, and 49%, respectively. In neither monotherapy nor combination therapy did differences between TCZ and ADA reach statistical significance.
Conclusion: Based on JIA ACR response rates from this analysis, the expected efficacy of ADA vs TCZ appears comparable in pcJIA. As monotherapy, however, TCZ may be better than ADA. These data should be interpreted in the context of differences in the duration of the withdrawal phase, which was shorter in the TCZ study (CHERISH) than in the ADA trial and might have resulted in a smaller difference in the number of flares observed between placebo and TCZ.
References: 1. Ruperto N et al. Lancet. 2008;372:383; 2. Lovell DJ et al. N Engl J Med. 2008;359:810; 3. Lovell DJ et al. N Engl J Med. 2000;342:763; 4. Ruperto N. Arthritis Rheum. 2007;56:3096; 5.Unpublished data from CHERISH.
Disclosure:
L. Sawyer,
Hoffmann-La Roche, Inc.,
5;
A. Diamantopoulos,
Hoffmann-La Roche, Inc.,
5;
H. Brunner,
Novartis, Genentech, MedImmune, EMD Serono, AMS, Pfizer, UCB, Janssen,
5,
Genentech ,
8;
F. De Benedetti,
Abbott, Pfizer, BMS, Roche, Novimmune, Novartis, SOBI,
2;
N. Ruperto,
Abbott, AstraZeneca, BMS, Centocor, Lilly, Francesco Angelini, GSK, Italfarmaco, MerckSerono, Novartis, Pfizer, Regeneron, Roche, Sanofi Aventis, Schwarz Biosciences, Xoma, Wyeth,
2,
Abbott, AstraZeneca, BMS, Centocor, Lilly, Francesco Angelini, GSK, Italfarmaco, MerckSerono, Novartis, Pfizer, Regeneron, Roche, Sanofi Aventis, Schwarz Biosciences, Xoma, Wyeth,
5,
Abbott, Boehringer, BMS, Novartis, Astellas, Italfarmaco, MedImmune, Pfizer, Roche,
8;
F. Dejonckheere,
F. Hoffmann-La Roche,
3;
C. Keane,
Roche Pharmaceuticals,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-biologic-treatments-in-juvenile-idiopathic-arthritis-with-a-polyarticular-course-an-indirect-comparison/