Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Behçet’s syndrome is a chronic, multi-system, variable vessel vasculitis characterized by painful, recurrent oral ulcers (OU) that can be disabling and may impair quality of life. Apremilast (APR), an oral phosphodiesterase 4 inhibitor, demonstrated efficacy in the treatment of OU of Behçet’s syndrome in a phase III, multinational, randomized, double-blind, placebo (PBO)-controlled study (RELIEF). Here, we report efficacy and safety of APR assessed over 64 weeks in the subgroup of Japanese patients in RELIEF.
Methods: Patients were randomized (1:1) to APR 30 mg BID or PBO BID for a 12-week PBO-controlled phase, followed by a 52-week active treatment extension. Patients were stratified by region (Japan/other). Eligible patients were ≥18 years of age, had active Behçet’s syndrome, with ≥3 OU at randomization or ≥2 OU at screening and randomization and without active major organ involvement. The primary efficacy endpoint was area under the curve for the number of OU over 12 weeks (AUCWk0-12). Clinical improvement in OU was evaluated by assessments of OU number and pain (100 mm visual analog scale), disease activity (Behçet’s Disease Current Activity Form [BDCAF], composed of the Behçet’s Disease Current Activity Index [BDCAI] and Patient’s and Clinician’s Perception of Disease Activity, and Behçet’s Syndrome Activity Score [BSAS]) over 64 weeks. An ANCOVA model was used to analyze the primary endpoint. In the Japanese subgroup, Week 12 variables were prespecified without multiplicity adjustment. Nominal P values are given. Data at Week 64 are as observed.
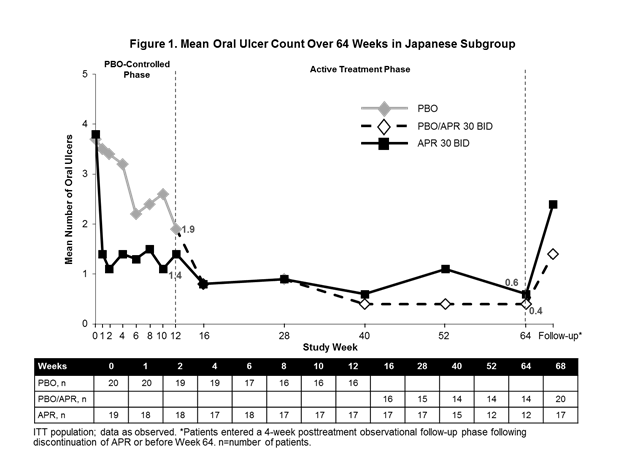
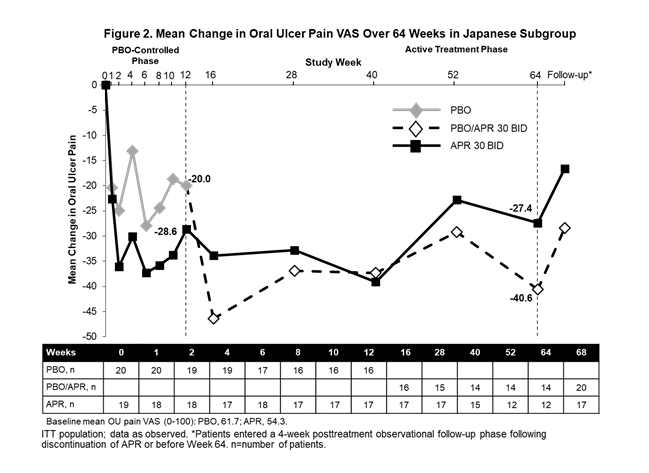
Results: Of the 207 randomized patients, 39 were Japanese (APR: n=19; PBO: n=20); 26 Japanese patients completed the active treatment phase (12 initially randomized to APR and 14 initially randomized to PBO). Consistent with the findings of the overall study population, the primary endpoint of AUCWk0-12 was significantly lower for APR vs PBO (115.9 vs 253.3; nominal P=0.0168). At Week 12, significantly more Japanese patients had OU complete response with APR (57.9%) vs PBO (25.0%) (nominal P=0.0426); numerically greater reductions in OU pain were observed with APR vs PBO (−17.0 [95% CI: −39.1, 5.1]; nominal P=0.1273). Improvements in mean number of OU (Figure 1), OU pain (Figure 2), complete and partial response rates, as well as disease activity assessments (BDCAF and BSAS) were observed at Week 12 and generally maintained in Japanese patients continuing APR 30 mg BID treatment for up to 64 weeks and emerged in those who switched from PBO to APR. At 4 weeks after APR treatment discontinuation, worsening in measures of oral ulcer disease was observed in both treatment groups. Adverse event (AE) rates were similar in the 2 groups during the PBO-controlled period (APR: 73.7%; PBO: 75.0%). Common AEs were diarrhea, upper respiratory tract infection, nausea, and headache. No new safety concerns were identified up to 64 weeks.
Conclusion: APR demonstrated efficacy in the treatment of OU in a subset of Japanese patients with Behçet’s syndrome from RELIEF. Benefits were sustained up to 64 weeks with continued APR treatment. Safety findings were consistent with the known AE profile of APR.
To cite this abstract in AMA style:
Takeno M, Tanaka Y, Kono H, Sugii S, Kishimoto M, Cheng S, McCue S, Chen M, Paris M, Dobashi H. Efficacy of Apremilast for Oral Ulcers Associated with Active Behçet’s Syndrome over 64 Weeks: Long-term Results from the Japanese Subgroup in a Phase III Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-apremilast-for-oral-ulcers-associated-with-active-behcets-syndrome-over-64-weeks-long-term-results-from-the-japanese-subgroup-in-a-phase-iii-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-apremilast-for-oral-ulcers-associated-with-active-behcets-syndrome-over-64-weeks-long-term-results-from-the-japanese-subgroup-in-a-phase-iii-study/