Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Results from the 1-year double-blind period and first year of the open-label extension (OLE) of the MANDARA trial (NCT04157348) demonstrated that over 60% of patients with relapsing or refractory eosinophilic granulomatosis with polyangiitis (EGPA), with active non-severe disease at baseline, achieved remission. Additionally, more than 40% discontinued oral glucocorticoids after treatment with anti-interleukin-5/Receptor (anti-IL-5/R) therapies. This analysis focuses on changes in individual items of active disease and long-term damage in EGPA over the same two-year period.
Methods: Patients (without active life- or organ-threatening manifestations at the start of the trial) who completed the randomized double-blind period, could enter the OLE, during which they continued benralizumab (benra/benra n=66) or switched from mepolizumab to benralizumab (mepo/benra n=62). The protocol encouraged reduction in oral glucocorticoids. Disease activity was measured by the Birmingham Vasculitis Activity Score (BVAS), while items of damage were measured by the Vasculitis Damage Index (VDI) for the trial participants over two years (up to Week 104).
Results: In total, 128 patients entered the OLE. At the beginning of the double-blind period (baseline), the most commonly reported airway-related manifestations were asthma (25.8% of patients), paranasal sinus involvement (15.6%), and bloody nasal discharge/crusts/ulcers/granulomata (14.1%). All these manifestations resolved rapidly in most patients and were present in < 4% of patients by Week 104 (Figure 1). Sensory peripheral neuropathy (9.4%), arthralgia/arthritis (7.8%), and myalgia (6.3%) were the most reported non-airway manifestations at baseline, and their frequency also decreased rapidly to < 3% by Week 104. Cutaneous (3.9%) and renal manifestations (1.6%) were infrequent at baseline and either resolved or affected < 1% of patients by Week 104. Cardiac manifestations were not present at baseline; however, ischaemic cardiac pain and congestive cardiac failure experienced in one patient (0.9%), were present at Week 60. Overall, there were 29 new items of damage in 25 patients recorded up to Week 104, with 18 occurring in the double-blind period, and 11 during the first year of the OLE (Figure 2).
Conclusion: Among patients with EGPA, after two years of treatment with anti-IL-5/R therapies, mostly associated with substantial reductions in oral glucocorticoids, there is little disease progressive organ damage, suggesting a durable effect of treatment with mepolizumab and benralizumab for reducing both airway-and non-airway-related manifestations of disease.
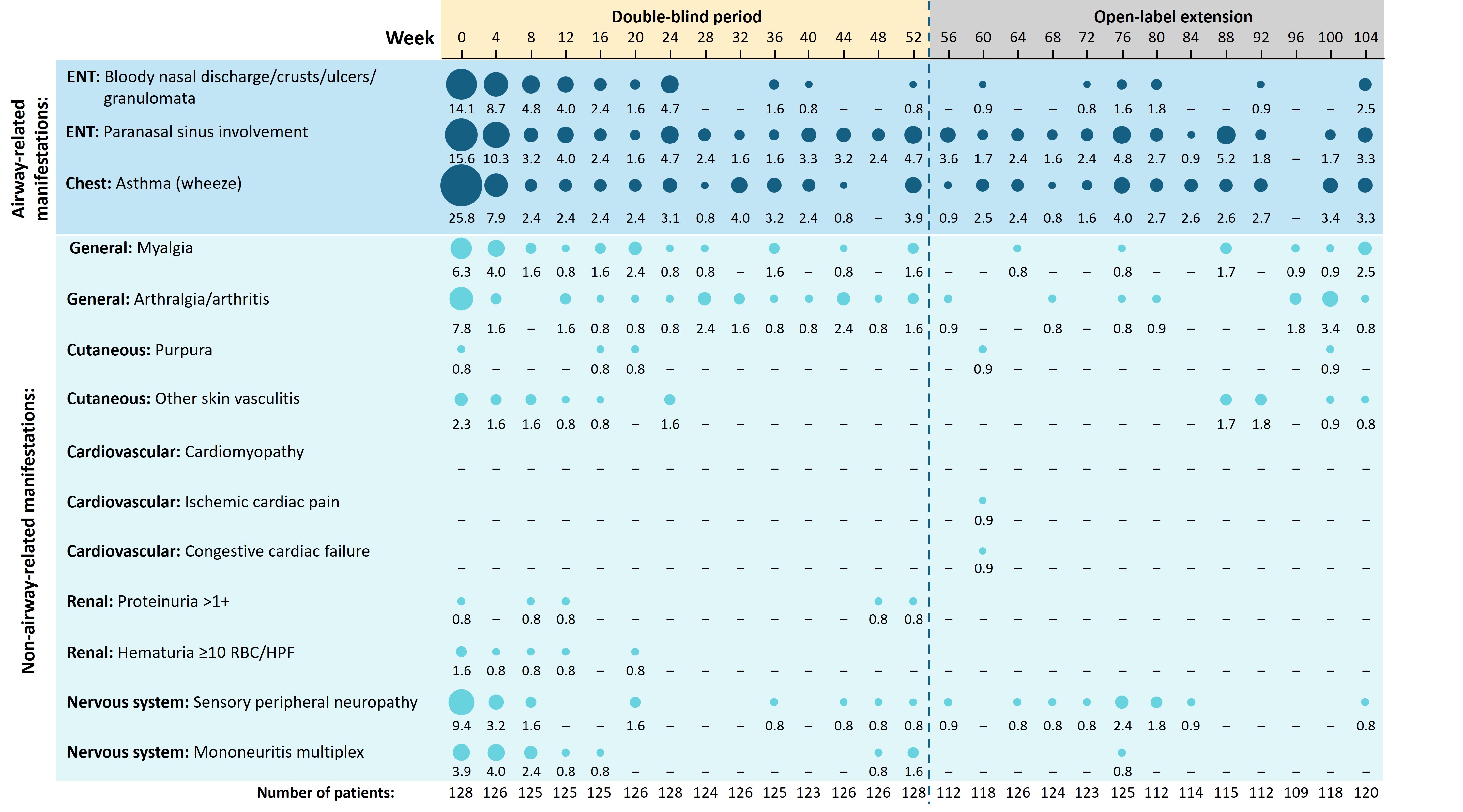 Figure 1. Summary of select manifestations of eosinophilic granulomatosis with polyangiitis up to Week 104 of the MANDARA trial
Figure 1. Summary of select manifestations of eosinophilic granulomatosis with polyangiitis up to Week 104 of the MANDARA trial
Corresponding percentage of patients is shown beneath each bubble. Manifestations are categorized according to the BVAS.
BVAS, Birmingham Vasculitis Activity Score; ENT, ear, nose, and throat; HPF, high power field; RBC, red blood cells.
.jpg) Figure 2. Summary of items of damage in patients with eosinophilic granulomatosis with polyangiitis up to Week 104 of the MANDARA trial
Figure 2. Summary of items of damage in patients with eosinophilic granulomatosis with polyangiitis up to Week 104 of the MANDARA trial
Corresponding percentage of patients is shown beneath each bubble, as measured by VDI.
ENT, ear, nose, and throat; VDI, Vasculitis Damage Index.
To cite this abstract in AMA style:
Merkel P, Bourdin A, Hellmich B, Khalidi N, Jackson D, Jayne D, Nair P, Pagnoux C, Specks U, Terrier B, Börjesson Sjö L, Jain P, Lal A, Necander S, Walton C, Wechsler M. Efficacy of Anti-IL-5/R Therapies on Specific Disease Manifestations of Eosinophilic Granulomatosis with Polyangiitis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-anti-il-5-r-therapies-on-specific-disease-manifestations-of-eosinophilic-granulomatosis-with-polyangiitis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-anti-il-5-r-therapies-on-specific-disease-manifestations-of-eosinophilic-granulomatosis-with-polyangiitis/
