Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: In the TULIP-2 and TULIP-1 trials of patients with SLE, the type I IFN receptor mAb anifrolumab resulted in higher BILAG–based Composite Lupus Assessment (BICLA) response rates vs placebo at Week 52.1,2 Subgroup analyses revealed concordant BICLA response rates across clinically distinct SLE subgroups, including disease severity and SLE therapies.3 Here, we compare BICLA response rates in serological subgroups (low complement, anti-dsDNA positivity, or both).
Methods: TULIP-2 (NCT02446899) and TULIP-1 (NCT02446912) were phase 3, randomized, placebo-controlled, 52-week trials of intravenous anifrolumab every 4 weeks for 48 weeks in eligible patients who fulfilled the ACR 1997 criteria for SLE and had moderate to severe SLE despite standard therapy.1,2 BICLA response rates at Week 52 for anifrolumab vs placebo groups were compared across patient subgroups of baseline complement C3/C4 levels (low/normal) and anti-dsDNA antibody status (positive/negative).
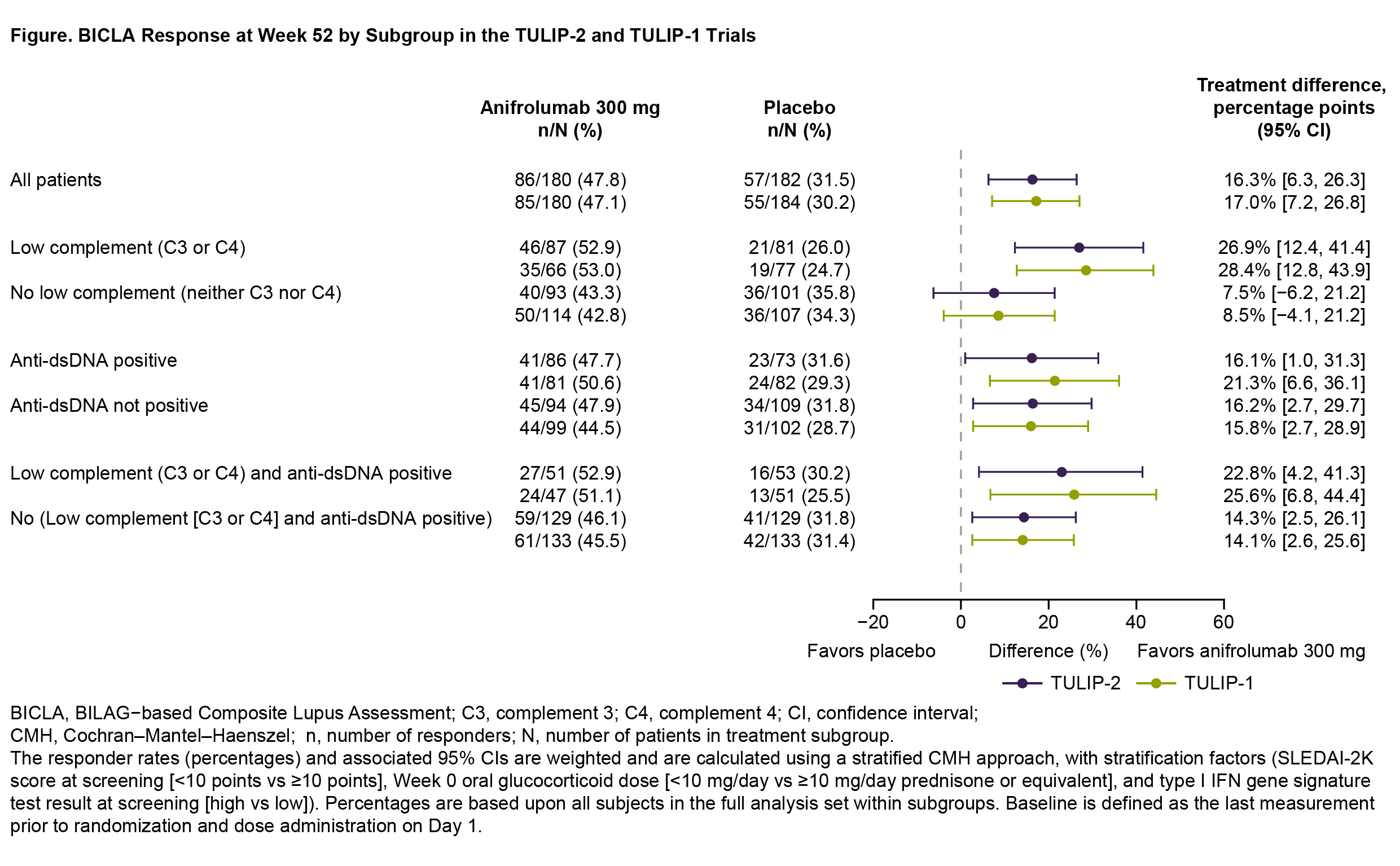
Results: In TULIP-2 and TULIP-1, 180 patients in each trial received anifrolumab 300 mg, and 182 and 184 patients received placebo, respectively. BICLA response rates in the overall anifrolumab groups were similar in TULIP-2 (47.8%) and TULIP-1 (47.1%), with treatment differences (Δ) favoring anifrolumab over placebo (Δ=16.3% and 17.0%, respectively) (Figure). Anifrolumab response rates were generally higher in serologically abnormal vs normal subgroups in TULIP-2 (47.7%–52.9% vs 43.3%–47.9%) and TULIP-1 (50.6%–53.0% vs 42.8%–45.5%); the greatest anifrolumab response rate was seen in the low C3/C4 subgroup (TULIP-2: 52.9%; TULIP-1: 53.0%), while anifrolumab response rates were similar regardless of anti-dsDNA positivity. In contrast, placebo response rates were generally lower in serologically abnormal vs normal subgroups in TULIP-2 (26.0%–31.6% vs 31.8%–35.8%) and TULIP-1 (24.7%–29.3% vs 28.7%–34.3%), although placebo responses were similar regardless of anti-dsDNA positivity. Anifrolumab and placebo subgroup response rates did not vary by more than ±6% from the overall population. These variations in response rates led to greater treatment differences favoring anifrolumab in patients with low C3/C4, alone or in combination with anti-dsDNA positivity, vs the overall population; the largest difference was seen for patients with low C3/C4 (TULIP-2: Δ=26.9%; TULIP-1: Δ=28.4%). In the subgroups with normal serology, treatment differences ranged from 7.5%–16.2% in TULIP-2 and 8.5%–15.8% in TULIP-1; thus, treatment differences favored anifrolumab vs placebo in all evaluated serology subgroups.
Conclusion: BICLA response rates in clinically distinct subgroups of SLE were generally consistent with the overall TULIP-2 and TULIP-1 results.3 Patients with low complement at baseline appeared to have a greater treatment effect than those with normal complement, while anti-dsDNA antibody status did not associate with response. The subgroup analyses indicate efficacy of anifrolumab 300 mg across all evaluated serological subgroups of SLE.
1. Furie, Lancet Rheumatol 2019;1:e208-10.
2. Morand. N Engl J Med 2020;382:211–21.
3. Morand. Ann Rheum Dis 2020(S1);79:32.
To cite this abstract in AMA style:
Bruce I, Van Vollenhoven R, Tanaka Y, Morand E, Furie R, Psachoulia K, Maho E, Lindholm C, Kleoudis C, Tummala R. Efficacy of Anifrolumab in Serological Subgroups of Patients with SLE Participating in 2 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-anifrolumab-in-serological-subgroups-of-patients-with-sle-participating-in-2-phase-3-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-anifrolumab-in-serological-subgroups-of-patients-with-sle-participating-in-2-phase-3-trials/