Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: In the phase 3 TULIP-1 and TULIP-2 trials, anifrolumab, a type I IFN receptor mAb, improved disease activity in patients with SLE.1,2 We investigated whether prior biologic exposure impacted anifrolumab efficacy and safety in TULIP-1 and TULIP-2 pooled data.
Methods: This analysis included patients who received intravenous anifrolumab 300 mg or placebo every 4 weeks for 48 weeks in the 52-week TULIP-1 (NCT02446912) and TULIP-2 (NCT02446899) trials, for which eligible patients met the ACR 1997 SLE criteria, had moderate to severe SLE, and were permitted prior biologic use with a 3−6 month washout period, regardless of the reason for cessation. Patients were split into biologic-experienced or biologic-naïve subgroups (≥1 or 0 previous biologic immunomodulators, respectively). Baseline SLE disease characteristics, efficacy, and safety were compared across subgroups. Efficacy measures included BILAG–based Combined Lupus Assessment (BICLA) response at Week (W) 52; SLE Responder Index of ≥4 (SRI[4]) response at W52; sustained oral glucocorticoid (GC) taper (≤7.5 mg/day prednisone equivalent, W40−52, if ≥10 mg/day at baseline); and annualized flare rate through W52. Binary endpoints and safety were analyzed with a Cochran–Mantel–Haenszel approach controlling for randomization stratification factors and study. Annualized flare rate was analyzed with a negative binomial regression model with treatment, randomization stratification factors, and study as covariates.
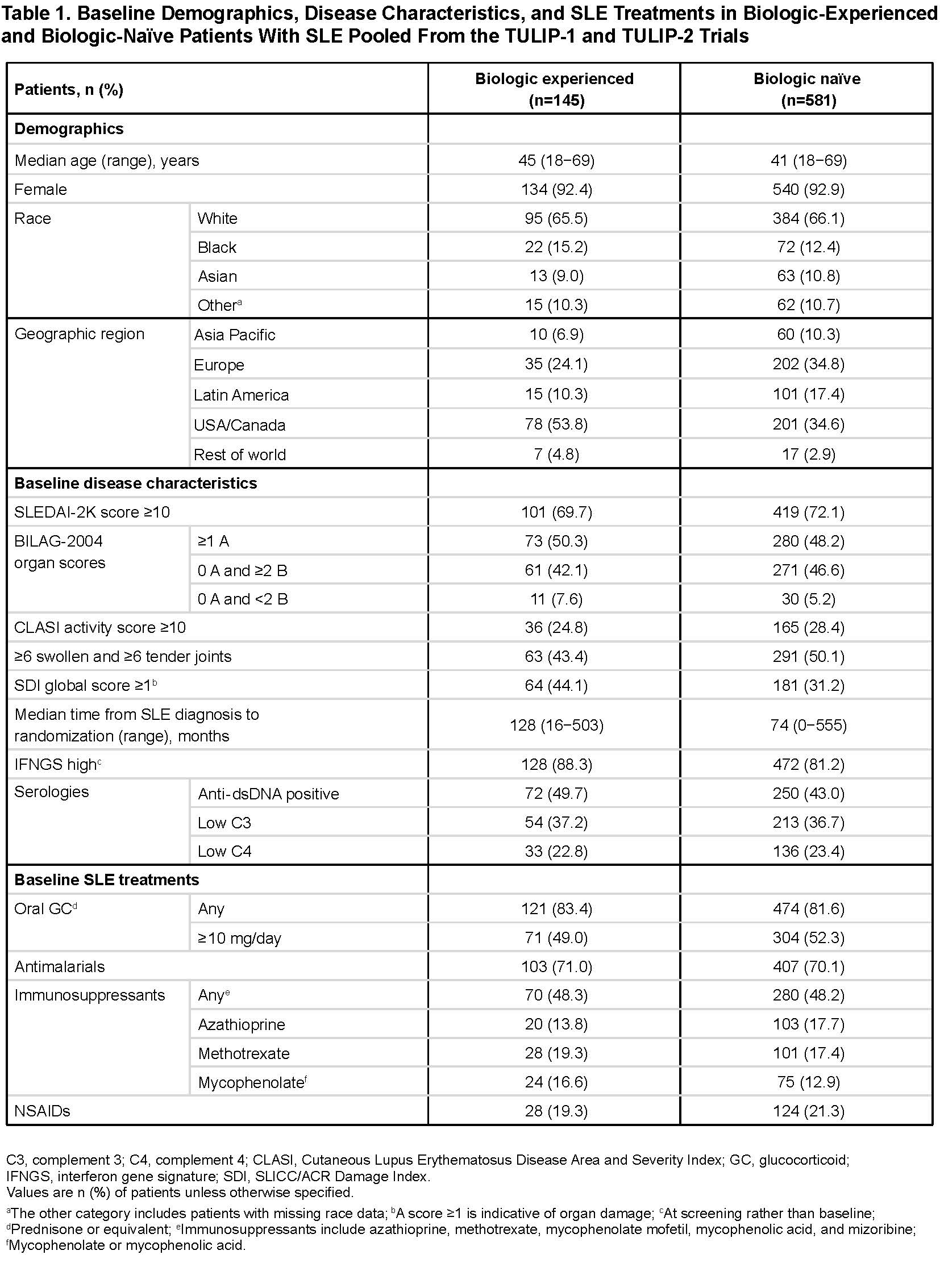
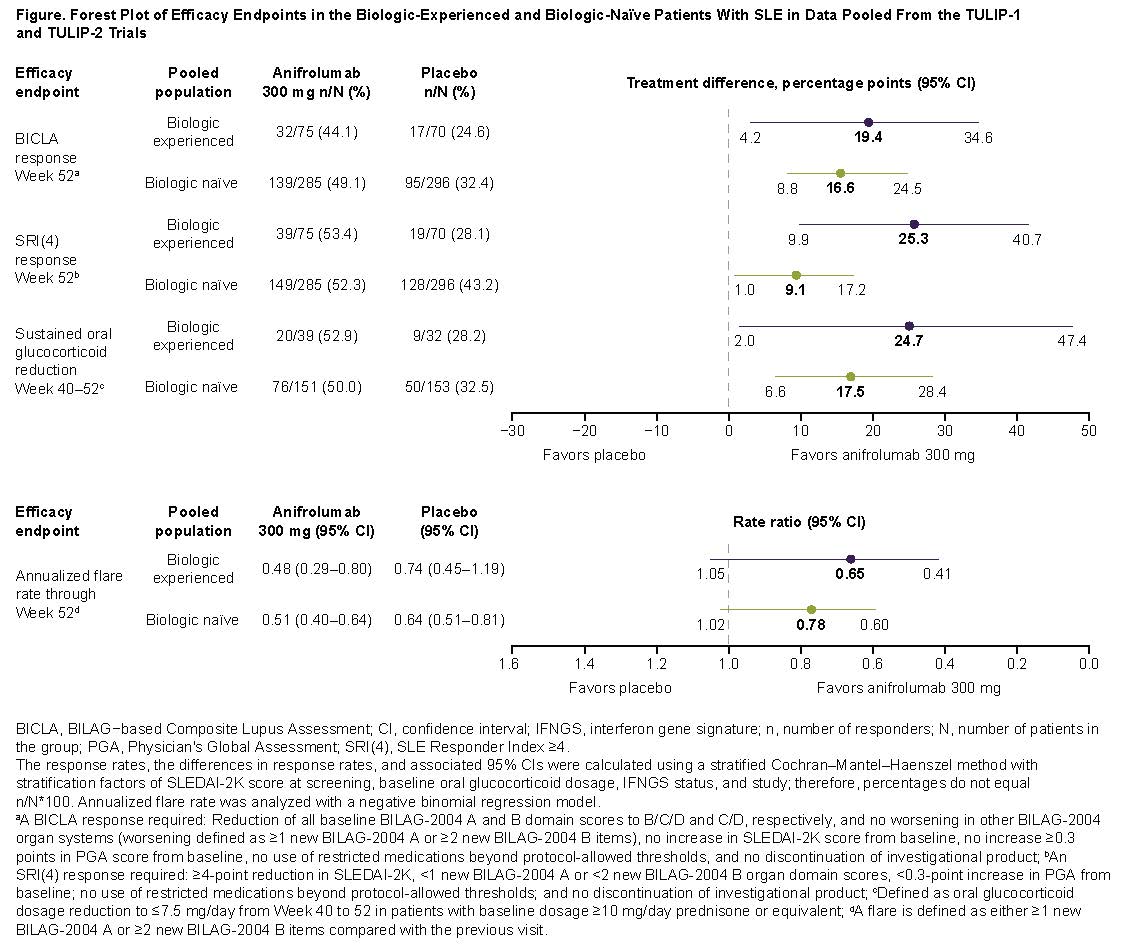
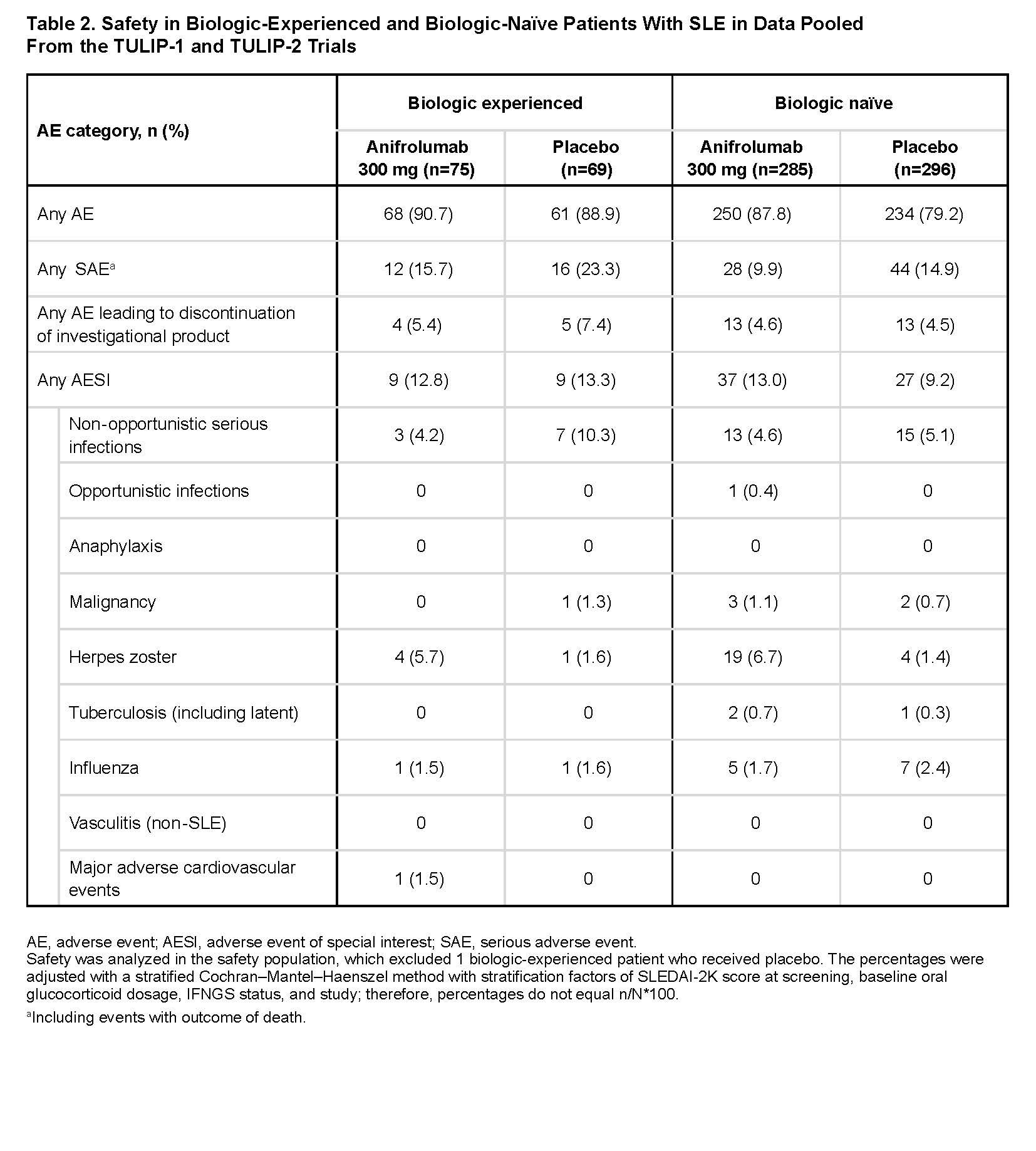
Results: There were 145 biologic-experienced patients (anifrolumab [n=75]; placebo [n=70]), and 581 biologic-naïve patients (anifrolumab [n=285]; placebo [n=296]). Most previous biologic use was with belimumab (n=70), epratuzumab (n=49), tabalumab (n=18), or rituximab (n=14). Baseline demographics, disease characteristics, and non-biologic SLE treatments were generally similar between groups. However, compared with biologic-naïve patients, biologic‑experienced patients had longer times from SLE diagnosis, were more likely to be from USA/Canada, have SLICC/ACR Damage Index score ≥1, anti‑dsDNA antibodies, and IFN gene signatures, and less likely to have swollen/tender joints (Table 1). There were lower placebo responses (potentially more refractory disease) in biologic‑experienced vs biologic-naïve patients (Figure). Anifrolumab was associated with comparable/greater treatment differences over placebo (∆) in biologic‑experienced vs biologic-naïve patients across endpoints, including BICLA (∆=19.4 vs ∆=16.6), SRI(4) (∆=25.3 vs ∆=9.1), and GC tapers (∆=24.7 vs ∆=17.5). Incidence of serious adverse events was higher in biologic‑experienced vs biologic-naïve patients with anifrolumab and placebo (Table 2). Herpes zoster incidence was higher with anifrolumab vs placebo in both biologic‑experienced and biologic-naïve patients.
Conclusion: Regardless of whether patients with SLE had previously received biologics, anifrolumab 300 mg provided clinically meaningful benefit over placebo across efficacy endpoints and was generally well tolerated.
1. Furie RA. Lancet Rheumatol. 2019;1:e208–19.
2. Morand EF. N Engl J Med. 2020;382:211–21.
To cite this abstract in AMA style:
Furie R, Morand E, Kalunian K, Psachoulia K, Maho E, Lindholm C, Tummala R. Efficacy of Anifrolumab in Patients with SLE Previously Treated with Biologics: Post Hoc Analysis of Data from 2 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-anifrolumab-in-patients-with-sle-previously-treated-with-biologics-post-hoc-analysis-of-data-from-2-phase-3-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-anifrolumab-in-patients-with-sle-previously-treated-with-biologics-post-hoc-analysis-of-data-from-2-phase-3-trials/