Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The response rate of TNFis in RA is variable and to some extent unpredictable, making treatment decision-making quite complex. Some insurance carriers require RA patients to have failed 2 different TNFi agents prior to a trial of a biologic agent with an alternate mechanism of action (MOA). This study retrospectively analyzed our 4282 RA patient database to evaluate patients who failed to respond adequately to an initial TNFi and look for clinical predictors of response to a 2nd TNFi.
Methods: A retrospective manual chart review of the electronic health record (EHR) was performed on 322 RA patients seen over the course of four years who were noted to have been prescribed more than one TNFi. Age, gender, body mass index (BMI), insurance provider, RA diagnosis length, cyclic citrullinated peptide antibody (CCP) and rheumatoid factor (RF) positivity, concomitant disease modifying anti-rheumatic drug (DMARD) therapy, length of time between diagnosis of RA and start of 1st and 2nd TNFi, efficacy of 1st and 2nd TNFi as evidenced by sustained or transient reduction in clinical disease activity index (CDAI) or impression of the treating rheumatologist, and reason for discontinuation were recorded. Patients who did and did not respond to their 1st and 2nd TNFi were compared using Pearson’s chi-square or Fisher’s exact tests and Student’s t-tests or Wilcoxon rank-sum tests. Comparisons of responses to 1st and 2nd TNFis were made with McNemar’s tests. A multivariable logistic regression model that included age, BMI, and statistically significant characteristics from the bivariate analysis was used to model response to a 2nd TNFi.
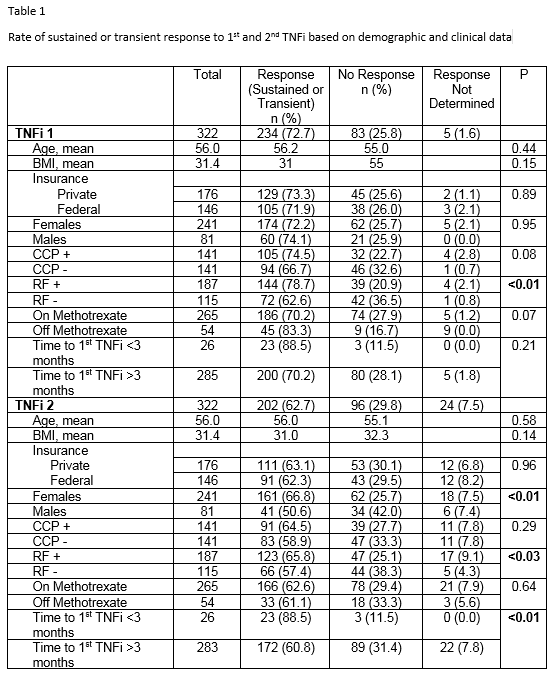
Results: Whether there was no response or transient achievement of response to a 1st TNFi as measured by CDAI or treating rheumatologist is displayed in Table 1. RF positive patients were more likely to respond than RF negative patients. Whether there was a transient response or not could not be determined in 5 patients (1.6%). Response rates to the 2nd TNFi are displayed in Table 1. Response to the 2nd TNFi could not be determined in 24 patients (7.5%). Response rates to the 2nd TNFi were greater in females vs males and in RF positive vs. RF negative patients. The predilection for female response was independent of age, BMI, and seropositivity. If the time to 1st TNFi was three months or less from initial diagnosis of RA, sustained response to 2nd TNFi was more likely compared to longer times.
Conclusion: In RA patients who failed to achieve or sustain a clinical response to an initial TNFi, female patients, patients with +RF, and patients whose diagnosis of RA was within three months of 1st TNF initiation were more likely to have a clinical response to a 2nd TNF agent. In the absence of these criterion, our data suggest a significantly reduced response to a 2nd TNFi . In these individuals, a stronger consideration for choosing a biologic with alternative MOA could be given.
To cite this abstract in AMA style:
Reams J, Berger A, Dunn P, O'Connell E, Torelli W, Bankert J, Bashir M, Denio A. Efficacy of a Second Tumor Necrosis Factor Inhibitor (TNFi) in the Treatment of Rheumatoid Arthritis (RA) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-a-second-tumor-necrosis-factor-inhibitor-tnfi-in-the-treatment-of-rheumatoid-arthritis-ra/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-a-second-tumor-necrosis-factor-inhibitor-tnfi-in-the-treatment-of-rheumatoid-arthritis-ra/