Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: SLE, an autoimmune disease affecting multiple organ systems, is characterized by periods of flares and remission. Type I interferon secreted at high levels by activated plasmacytoid dendritic cells (pDCs) drives downstream SLE disease activity. Daxdilimab (DAX) is a human mAb that targets pDC-specific cell surface protein immunoglobulin-like transcript 7 via antibody-dependent cellular cytotoxicity. Here, we report efficacy and safety results from a phase 2, randomized, placebo (PBO)-controlled trial of DAX in patients (pts) with SLE (NCT04925934).
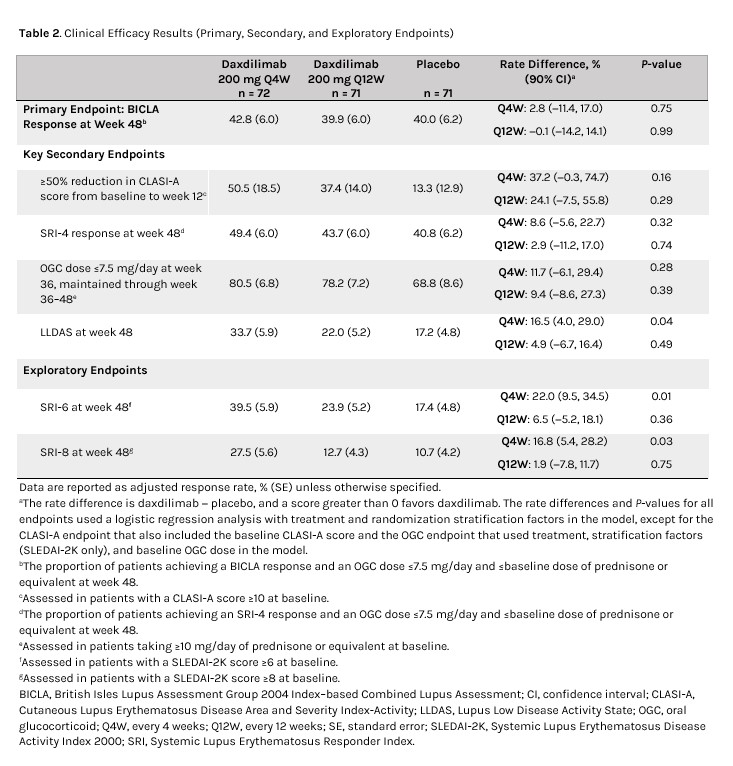
Methods: Pts were 18 to 70 years old (inclusive), fulfilled the 2019 EULAR/ACR SLE classification criteria, and received ongoing treatment. Pts were randomized 1:1:1 to DAX 200 mg subcutaneously every 4 weeks (Q4W), every 12 weeks (Q12W), or PBO Q4W for 48 weeks (W). The primary endpoint was the proportion of pts achieving a British Isles Lupus Assessment Group 2004 Index–based Combined Lupus Assessment (BICLA) response and an oral glucocorticoid dose ≤7.5 mg/day and ≤baseline dose (prednisone or equivalent) at W48.
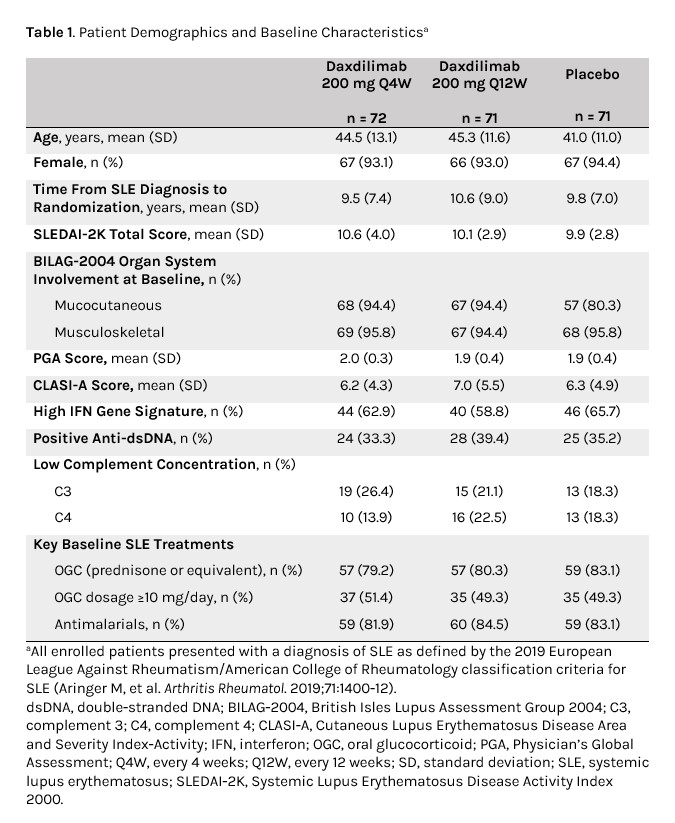
Results: The trial included 214 pts (DAX Q4W, n = 72; DAX Q12W, n = 71; PBO, n = 71). A total of 45 (21.0%) pts discontinued (most common reasons: withdrawal by pt, n = 22 [10.3%]; other, n = 18 [8.4%]; lost to follow-up, n = 3 [1.4%]). Baseline characteristics were comparable among groups (Table 1). At W48, blood pDC levels (cells/μL) decreased after treatment with DAX vs PBO (median percent change from baseline: −65.2%, −39.2%, and 16.7% for DAX Q4W, Q12W, and PBO, respectively).
There was no significant difference between DAX Q4W or Q12W vs PBO for the primary endpoint at W48 (Table 2), driven by the notably high response rate in the PBO group. More pts achieved Lupus Low Disease Activity State (LLDAS) with DAX Q4W vs PBO at W48; adjusted response rate (SE) was 33.7% (5.9%; P = 0.04), 22.0% (5.2%; P = 0.49), and 17.2% (4.8%) for DAX Q4W, Q12W, and PBO, respectively. No treatment differences were observed between DAX and PBO for other secondary endpoints. Positive trends were observed at W48 favoring DAX vs PBO for exploratory endpoints SLEDAI-2K, SLE Responder Index (SRI)-6, and SRI-8 (Table 2).
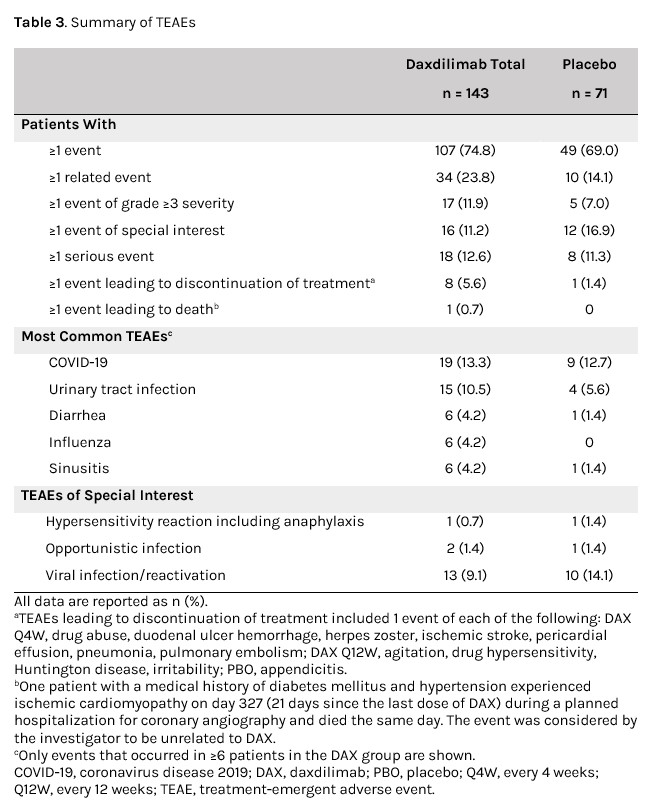
Treatment-emergent adverse events (TEAEs) occurred in 107/143 (74.8%) vs 49/71 (69.0%) pts who received DAX vs PBO (Table 3). AEs of special interest occurred in 16/143 (11.2%) and 12/71 (16.9%) pts who received DAX vs PBO, while serious AEs were reported in 18/143 (12.6%) and 8/71 (11.3%) pts, respectively. In the DAX and PBO groups, 8/143 (5.6%) and 1/71 (1.4%) pts, respectively, experienced ≥1 TEAE leading to treatment discontinuation; 1 death occurred (ischemic cardiomyopathy; unrelated to DAX).
Conclusion: DAX did not show a significant difference vs PBO in the primary endpoint, driven by the high response rate in the PBO group (Furie P, et al. Arthritis Rheumatol. 2017;69:376-86). However, a greater proportion of pts who received DAX Q4W achieved LLDAS at W48 vs PBO; a positive trend was observed with DAX vs PBO for other secondary/exploratory endpoints, suggesting a potential treatment effect. DAX was generally well tolerated. Depletion of pDC levels by DAX has prompted continued investigation in other autoimmune diseases like primary DLE and DM.
To cite this abstract in AMA style:
Khosroshahi A, Welsh S, Wang L, Larson D, Jain N. Efficacy and Safety Results from a Phase 2 Trial of Daxdilimab in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-results-from-a-phase-2-trial-of-daxdilimab-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-results-from-a-phase-2-trial-of-daxdilimab-in-patients-with-systemic-lupus-erythematosus/