Session Information
Date: Monday, October 22, 2018
Title: 4M089 ACR Abstract: Spondyloarthritis Incl PsA–Clinical III: Treatment of Axial SpA (1864–1869)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Methods:
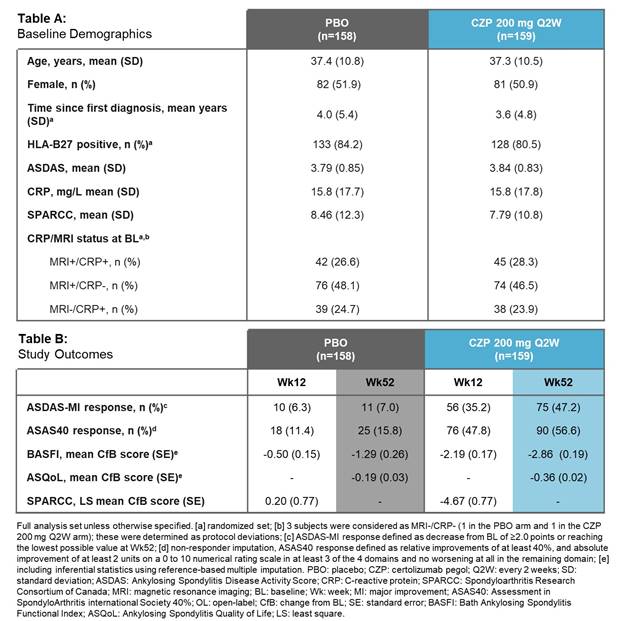
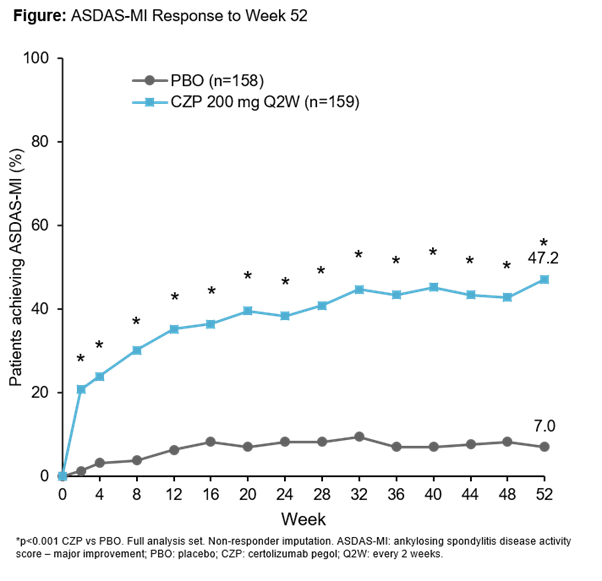
C-axSpAnd (NCT02552212) was a 52-wk, phase 3, multicenter, double-blind, PBO-controlled study. Pts were randomized 1:1 to PBO or CZP (400 mg at Weeks 0, 2, and 4, then 200 mg every 2 wks) and stratified by sacroiliitis on MRI and C-reactive protein (CRP) at baseline (BL) and region. Pts were ≥18 years with OSI (elevated CRP and/or positive MRI of the sacroiliac [SI] joint), symptom duration ≥12 months, documented diagnosis of axSpA and met ASAS (but not modified New York) classification criteria. Randomized pts could switch to open-label (OL) CZP treatment or alternative OL treatment at any time, and concomitant medication could be adjusted at any point during the trial. The primary efficacy variable was Ankylosing Spondylitis Disease Activity Score Major Improvement (ASDAS-MI; defined as ASDAS decrease from BL ≥2.0 points or reaching lowest possible value) at Wk52. ASAS40 Wk12 response was assessed as the first secondary variable.
This study was funded by UCB Pharma, medical writing by Eleanor Thurtle, Costello Medical, UK.
To cite this abstract in AMA style:
Deodhar AA, Gensler LS, Kay J, Maksymowych WP, Haroon N, Landewé RBM, Rudwaleit M, Hall S, Bauer L, Hoepken B, de Peyrecave N, Kilgallen B, van der Heijde D. Efficacy and Safety Outcomes in Patients with Non-Radiographic Axial Spondyloarthritis Treated with Certolizumab Pegol: Results from the First 52-Week Randomized Placebo-Controlled Study (NCT02552212) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-outcomes-in-patients-with-non-radiographic-axial-spondyloarthritis-treated-with-certolizumab-pegol-results-from-the-first-52-week-randomized-placebo-controlled-study-nct02552212/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-outcomes-in-patients-with-non-radiographic-axial-spondyloarthritis-treated-with-certolizumab-pegol-results-from-the-first-52-week-randomized-placebo-controlled-study-nct02552212/