Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
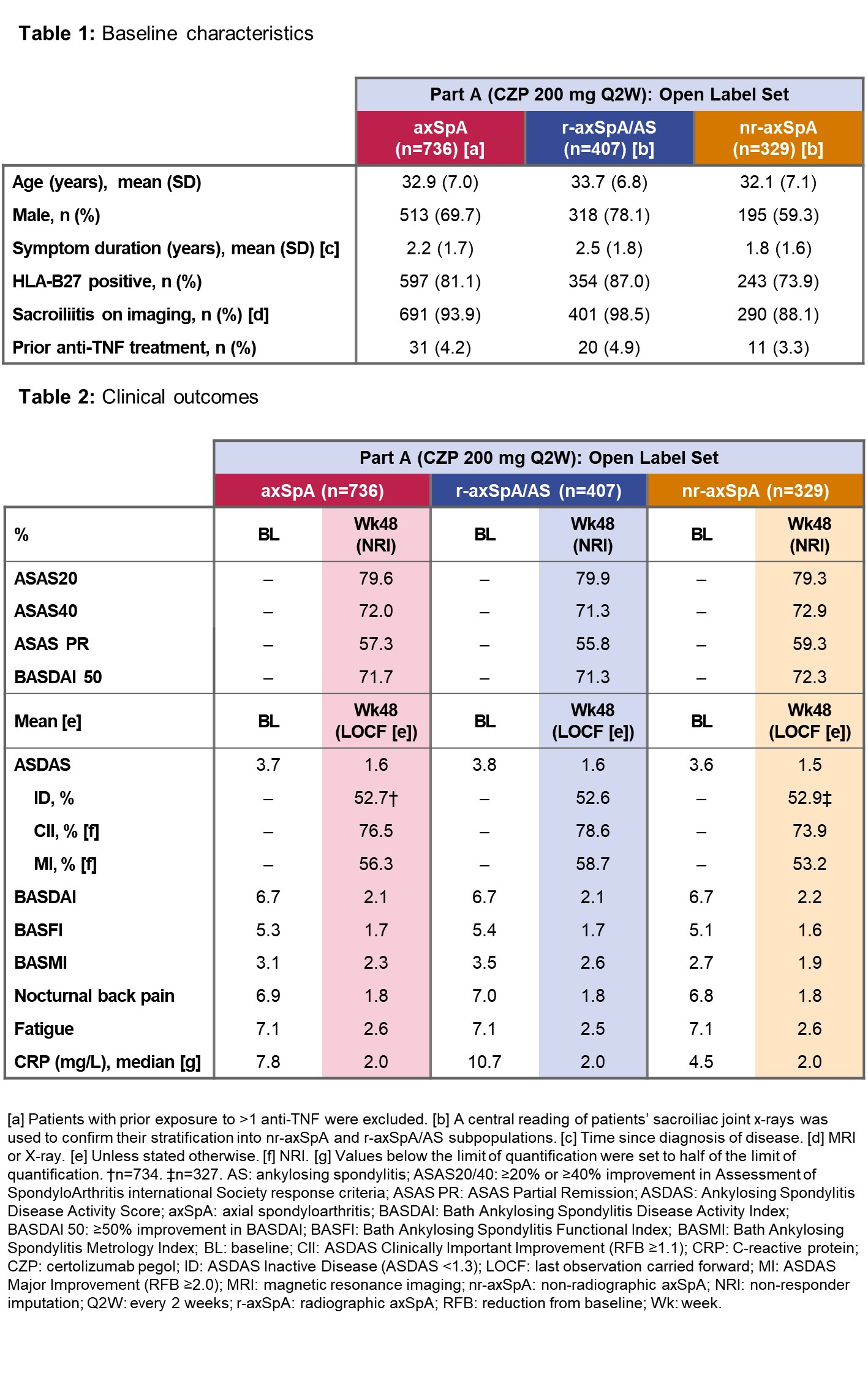
C-OPTIMISE is the first trial to evaluate whether certolizumab pegol (CZP) can be reduced/discontinued in patients with radiographic(r)-axSpA/ankylosing spondylitis (AS) and non-radiographic(nr)-axSpA achieving sustained remission after 48 weeks’ (wks’) treatment. Here, we report interim efficacy and safety data for both subpopulations from the ongoing trial.
Methods:
Up to wk48, C-OPTIMISE (NCT02505542) was open-label (Part A), followed by 48-wk parallel-group, double-blind, placebo-controlled treatment (full dose and half dose) to wk96 (Part B). Patients with adult-onset axSpA of <5 years’ duration, fulfilling ASAS classification criteria, were recruited. Part A: patients received CZP (400mg at wks0/2/4, then 200mg Q2W); patients achieving sustained remission (ASDAS<1.3 at wk32 and <2.1 at wk36 [or vice versa], and <1.3 at wk48) were eligible for Part B (secondary outcome). Primary outcome (not reported): percentage of patients in Part B not experiencing a flare. Missing values were imputed using non-responder imputation (NRI) and last observation carried forward (LOCF).
Results:
Part A: Of 736 patients (Table 1), 43.9% achieved sustained remission (r-axSpA/AS: 42.8%; nr-axSpA: 45.3%; NRI). At baseline, 98.5% patients had high/very high disease activity (ASDAS≥2.1); at Wk48, 52.7% (r-axSpA/AS: 52.6%; nr-axSpA: 52.9%) had inactive disease (ASDAS<1.3; LOCF; Table 2). The treatment-emergent adverse event (TEAE) rate/100 patient-years’ exposure was 224.2; 3.9% patients discontinued CZP due to TEAEs. No new safety signal was identified.
Conclusion:
The run-in phase of C-OPTIMISE shows that similar and substantial proportions of patients with r-axSpA/AS and nr-axSpA achieved sustained remission during 48 wks’ CZP treatment. No new safety signal was identified.
The study was funded by UCB Pharma, medical writing by Hinal Tanna, Costello Medical, UK. We thank the patients who contributed.
To cite this abstract in AMA style:
Landewé RBM, van der Heijde D, Dougados M, Baraliakos X, van Den Bosch F, Hoepken B, Thomas K, Gensler LS. Efficacy and Safety Outcomes in Patients with Axial Spondyloarthritis Treated with Certolizumab Pegol: Results from the 48-Week Run-in Part of a Withdrawal Study (NCT02505542) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-outcomes-in-patients-with-axial-spondyloarthritis-treated-with-certolizumab-pegol-results-from-the-48-week-run-in-part-of-a-withdrawal-study-nct02505542/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-outcomes-in-patients-with-axial-spondyloarthritis-treated-with-certolizumab-pegol-results-from-the-48-week-run-in-part-of-a-withdrawal-study-nct02505542/