Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: For patients with RA who are refractory to biologic disease-modifying antirheumatic drugs (bDMARDs), such as tumor necrosis factor inhibitors (TNFis), optimal disease control is less likely to be achieved with subsequent therapy.1 In line with recommendations from EULAR and ACR, switching to a treatment with a different mechanism of action is appropriate for these patients.
We describe the efficacy and safety of upadacitinib (UPA) 15 mg once daily in patients with RA and an inadequate response or intolerance to TNFis (TNFi-IR).
Methods: A post hoc subgroup analysis was conducted in TNFi-IR patients who were treated with UPA 15 mg once daily in three Phase 3 clinical trials: SELECT-BEYOND,2 -CHOICE,3 and -COMPARE.4 For COMPARE, only patients treated with adalimumab and switched to UPA as rescue therapy were included. ≥20/50/70% improvement in ACR criteria, DAS28-CRP, Clinical Disease Activity Index, and Simple Disease Activity Index, as well as change from baseline in HAQ-DI and other patient-reported outcomes (PROs) were reported through 24 weeks. Non-responder imputation was used for all missing categorical outcomes; as observed (COMPARE) or multiple imputation (CHOICE, BEYOND) were used for missing continuous outcomes. Pooled safety results were presented as exposure-adjusted event rates (EAERs) with a cut-off of June 30, 2021.
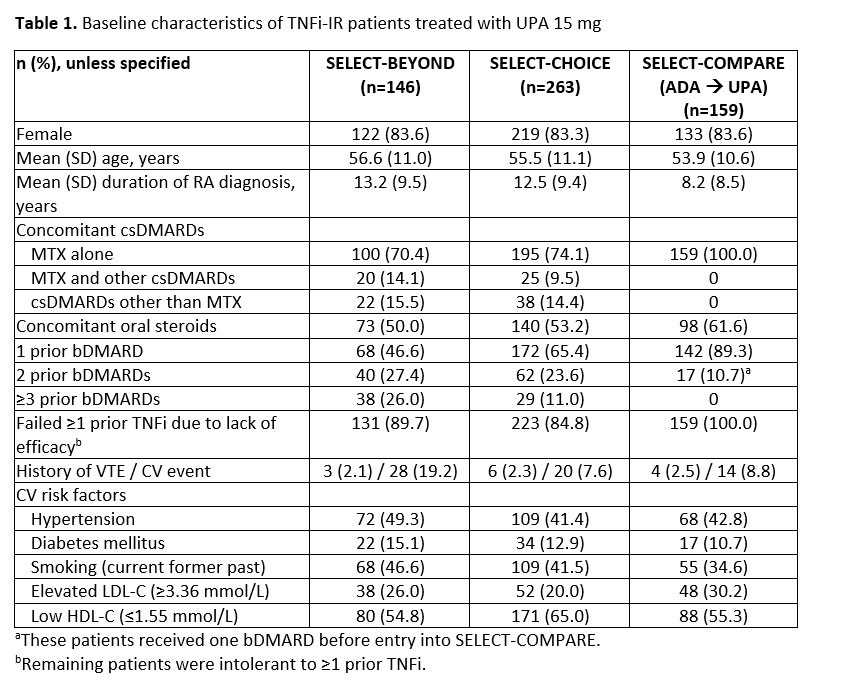
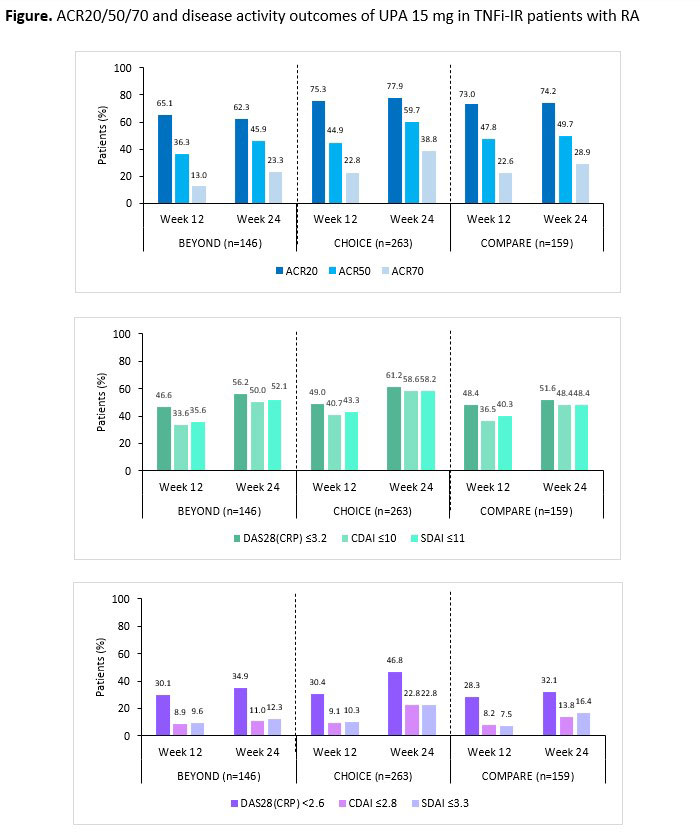
Results: 568 TNFi-IR patients were included: 146 from BEYOND, 263 from CHOICE, and 159 from COMPARE. Mean duration since RA diagnosis was longer for BEYOND and CHOICE versus COMPARE; CV risk factors were common among this refractory population (Table). ACR20/50/70 and disease activity outcomes observed in the TNFi-IR population were generally consistent with the overall BEYOND2 and CHOICE3 bDMARD-IR populations, and consistent across the three studies in the TNFi-IR subgroups (Figure). Improvements in PROs including HAQ-DI, fatigue, pain, and morning stiffness over 24 weeks were observed (data not shown). Pooled safety results reporting 1574.8 PY of exposure in the TNFi-IR subgroup showed similar results to the overall BEYOND2 and CHOICE3 bDMARD-IR study populations, with EAERs of 3.1 events/100 PY for herpes zoster and 0.8 events/100 PY for adjudicated major adverse CV events and venous thromboembolism, and malignancy excluding non-melanoma skin cancer. The EAER of any AE leading to death was 1.4 events/100 PY.
Conclusion: In this post hoc subgroup analysis, TNFi-IR patients treated with UPA 15 mg achieved clinically meaningful efficacy responses over 24 weeks, with safety consistent with the overall bDMARD-IR patient population in the Phase 3 program.
References: 1. Rendas-Baum R, et al. Arthritis Res Ther 2011;13:R25; 2. Genovese C, et al. Lancet 2018;391:2513–24; 3. Rubbert-Roth A, et al. NEJM 2020;383:1511–21;
4. Fleischmann R, et al. Ann Rheum Dis 2019;78:1454–62.
To cite this abstract in AMA style:
Fleischmann R, Bessette L, Sparks J, Hall S, Jain M, Kakehasi A, Song Y, Meerwein S, DeMasi R, Suboticki J, Rubbert-Roth A. Efficacy and Safety of Upadacitinib in TNFi-IR Patients with Rheumatoid Arthritis from Three Phase 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-tnfi-ir-patients-with-rheumatoid-arthritis-from-three-phase-3-clinical-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-tnfi-ir-patients-with-rheumatoid-arthritis-from-three-phase-3-clinical-trials/