Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Upadacitinib (UPA), an oral Janus kinase inhibitor, has been evaluated in the treatment of AS both in patients (pts) naïve to biologic DMARDs (bDMARDs)1 and in those with an inadequate response (IR; defined as intolerance to and/or lack of efficacy [LoE] of) to bDMARDs (bDMARD-IR).2 This post hoc analysis evaluated the efficacy and safety of UPA in selected subgroups of pts with bDMARD-IR AS based on experience with prior therapy.
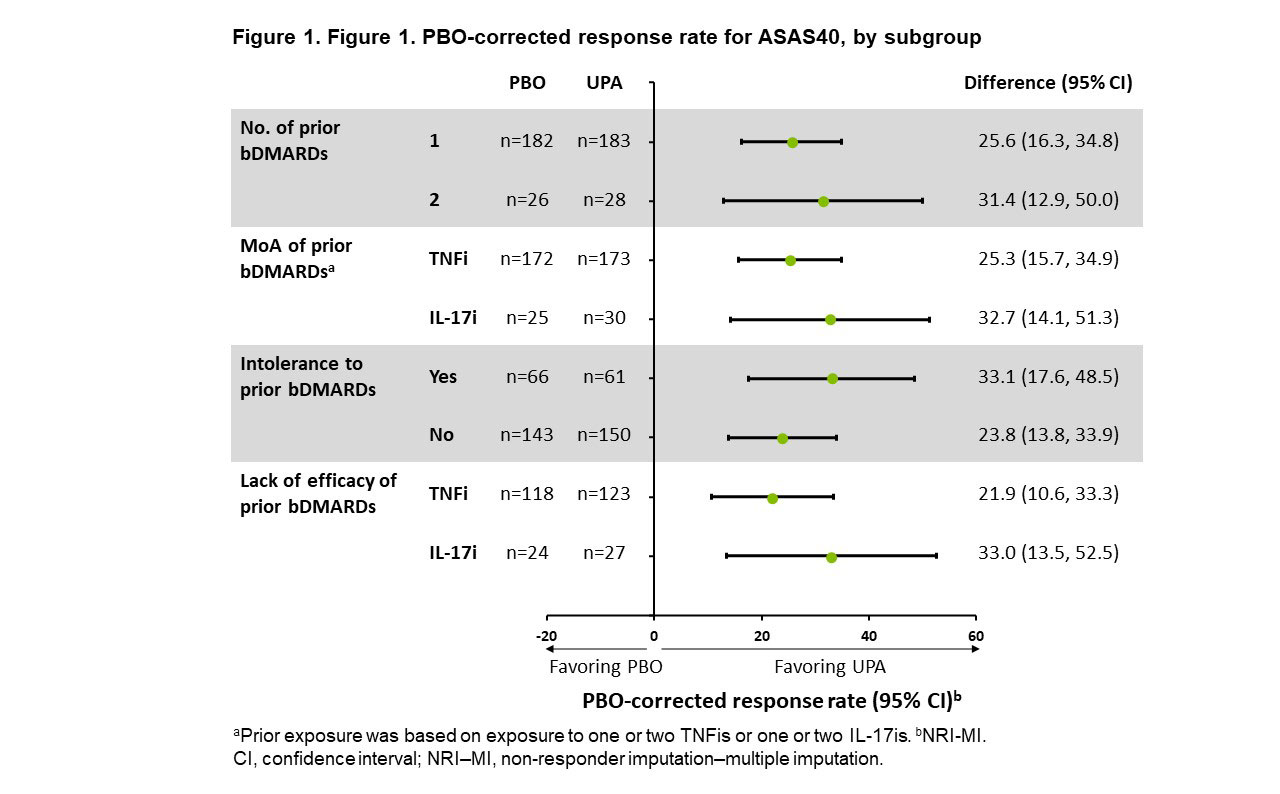
Methods: In the ongoing Phase 3 SELECT-AXIS 2 trial (NCT04169373), pts with bDMARD-IR AS who met the modified New York criteria for AS (N=420) were randomized 1:1 to receive UPA 15 mg once daily (n=211) or placebo (PBO; n=209) for 14 weeks (wks). Eligibility criteria included LoE after ≥12 wks of treatment with 1 prior bDMARD (TNF or IL-17 inhibitor [TNFi or IL-17i]) and/or intolerance to 1 or 2 prior bDMARD(s) regardless of treatment duration. LoE of 2 bDMARDs was not permitted. The primary study endpoint was Assessment of SpondyloArthritis international Society score (ASAS)40 response at Wk 14. This post hoc analysis evaluated ASAS40 and the following secondary endpoints, all at Wk 14, in subgroups determined by prior therapy: low disease activity (defined as ASDAS using CRP [ASDAS-CRP LDA] < 2.1), and improvement from baseline (BL) in pts’ assessment of total back pain, BASFI, and Spondyloarthritis Research Consortium of Canada (SPARCC) MRI spine inflammation score. Subgroup analyses were performed by number (1 or 2), mode of action (TNFi or IL-17i), and LoE (TNFi or IL-17i) of, and intolerance to (yes or no), prior bDMARDs.
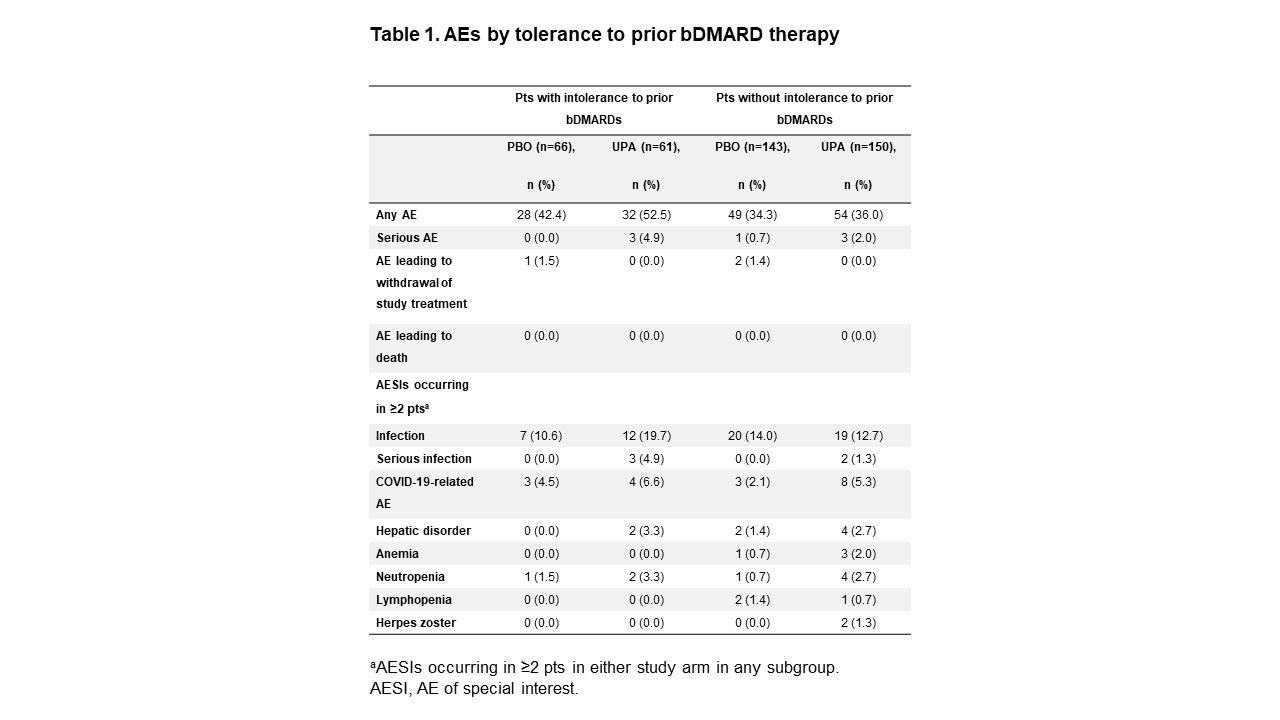
Results: BL characteristics were similar in the UPA and PBO groups.2 The majority of pts had prior exposure to 1 TNFi (UPA: 73%, PBO: 76%); 4% and 5% of pts receiving UPA and PBO, respectively, had prior exposure to both 1 TNFi and 1 IL-17i. ASAS40 response rates were increased with UPA vs PBO (difference range: 22–33%) at Wk 14 across all subgroups examined (Figure 1). ASDAS-CRP LDA response and improvement from BL in BASFI, pts’ assessment of total back pain, and SPARCC MRI spine inflammation score were also increased with UPA vs PBO in all subgroups (difference range: 28–37%,1.0–1.6, 1.3–2.3, and 0.8–4.8, respectively) (Figure 2). Adverse event (AE) rates were higher with UPA vs PBO regardless of tolerance to prior bDMARDs; however, AEs leading to the withdrawal of study treatment were similar in pts with vs without intolerance to prior bDMARDs (0% vs 1.5% and 0% vs 1.4%, respectively, with UPA vs PBO) (Table 1). AEs, serious AEs (SAEs), infection, and serious infection rates were higher in UPA recipients with vs without intolerance to prior bDMARDs (52% vs 36%, 5% vs 2%, 20% vs 13%, and 5% vs 1%, respectively). No deaths occurred.
Conclusion: In SELECT-AXIS 2, UPA demonstrated improved efficacy vs PBO at Wk 14 across all evaluated subgroups of pts with bDMARD-IR AS. The safety profile of UPA was consistent with the overall study findings, regardless of tolerance to prior bDMARDs. While the ability to draw conclusions is limited by the small size of some subgroups, UPA demonstrated a generally favorable benefit–risk profile in pts with AS refractory to prior advanced therapies.
References 1. van der Heijde D, et al. Lancet 2019;394:2108–17; 2. van der Heijde D, et al. Ann Rheum Dis 2022;81(Suppl 1):402–3
To cite this abstract in AMA style:
Baraliakos X, Ganz F, Kameda H, Walsh J, Jain M, D’Silva K, Wung P, Bu X, Stigler J, van der Heijde D. Efficacy and Safety of Upadacitinib in Patients with Ankylosing Spondylitis with Intolerance to And/or Lack of Efficacy of Prior Biologic Therapy: A Subgroup Analysis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-ankylosing-spondylitis-with-intolerance-to-and-or-lack-of-efficacy-of-prior-biologic-therapy-a-subgroup-analysis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-ankylosing-spondylitis-with-intolerance-to-and-or-lack-of-efficacy-of-prior-biologic-therapy-a-subgroup-analysis/