Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: RA affects diverse patient populations with varying disease severity and treatment responses. However, data on treatment response to advanced therapies by race are limited.1 Upadacitinib (UPA), an oral Janus kinase inhibitor, has demonstrated a favorable benefit–risk profile in patients with RA across the Phase 3 SELECT trials. This post hoc analysis evaluated the efficacy and safety of UPA in patients by race in these trials.
Methods: Data from patients who received UPA 15 mg once daily in SELECT-EARLY, -MONOTHERAPY, -NEXT, -BEYOND, -COMPARE, and ‑CHOICE were pooled. Patients were stratified by self-reported race into four groups: White, Black or African American (BAA), Asian, or Other. Efficacy endpoints at Week 12 and long-term exposure-adjusted adverse event (AE) rates stratified by race were evaluated.
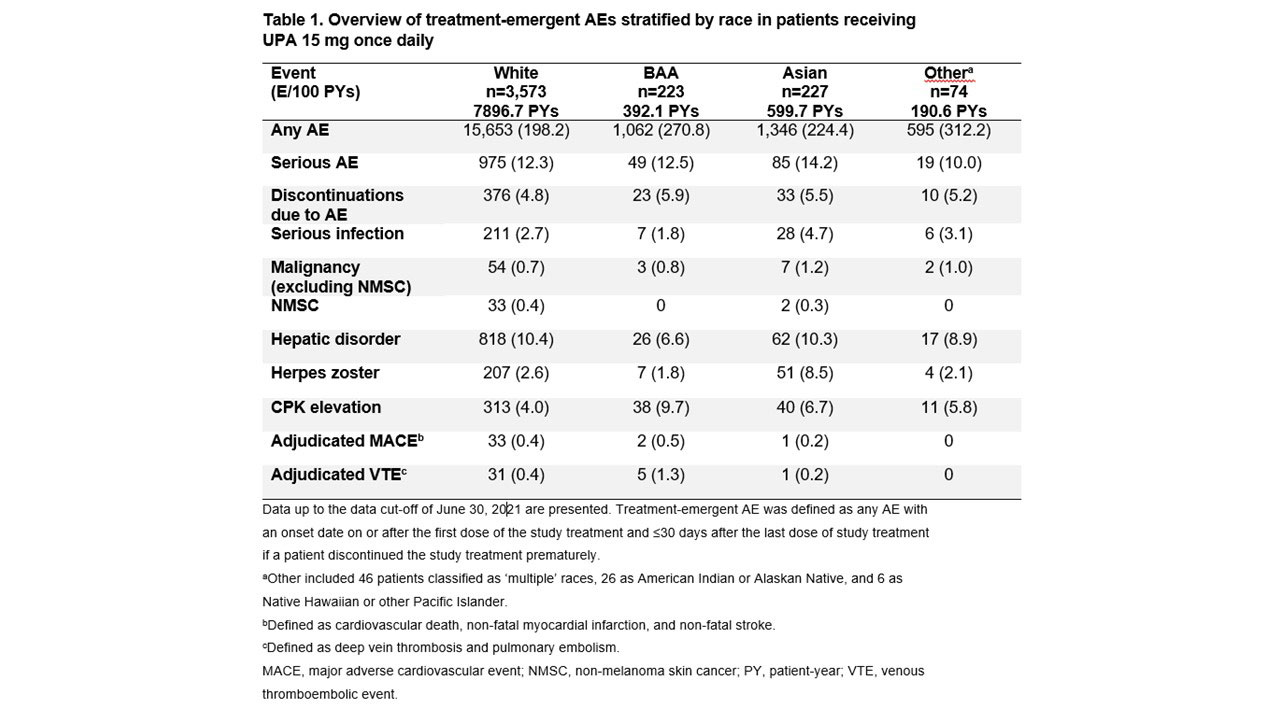
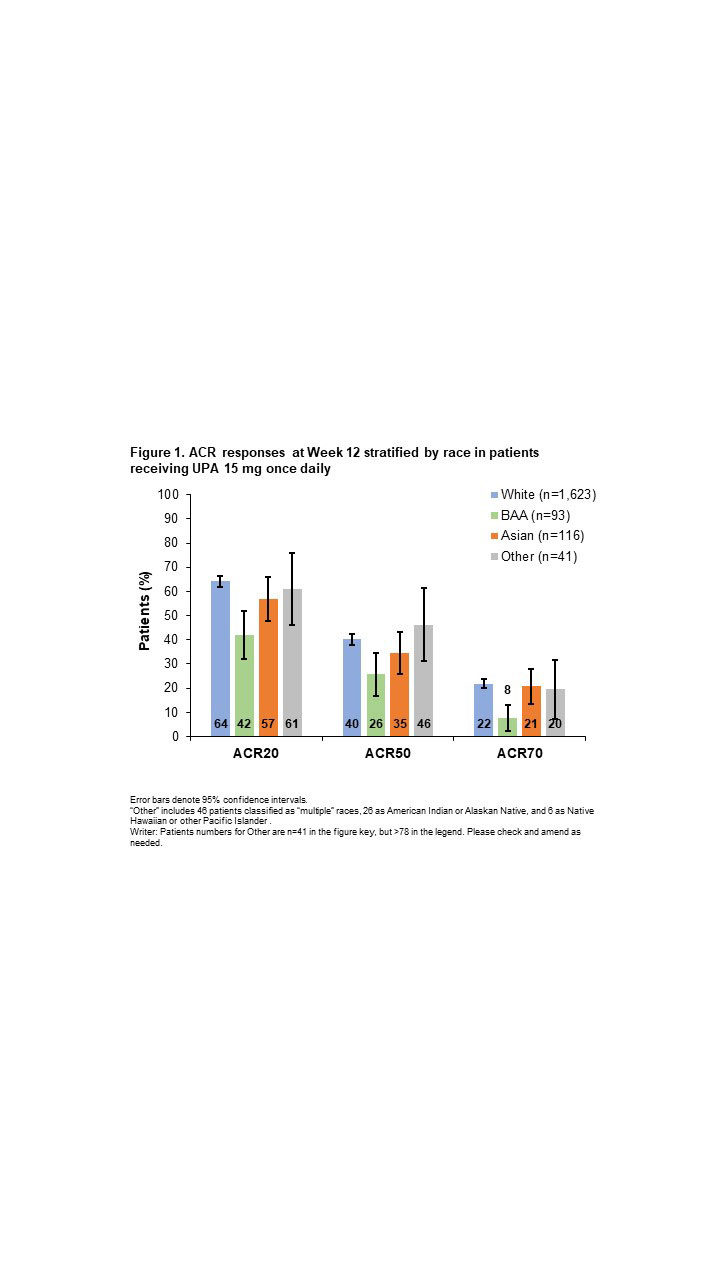
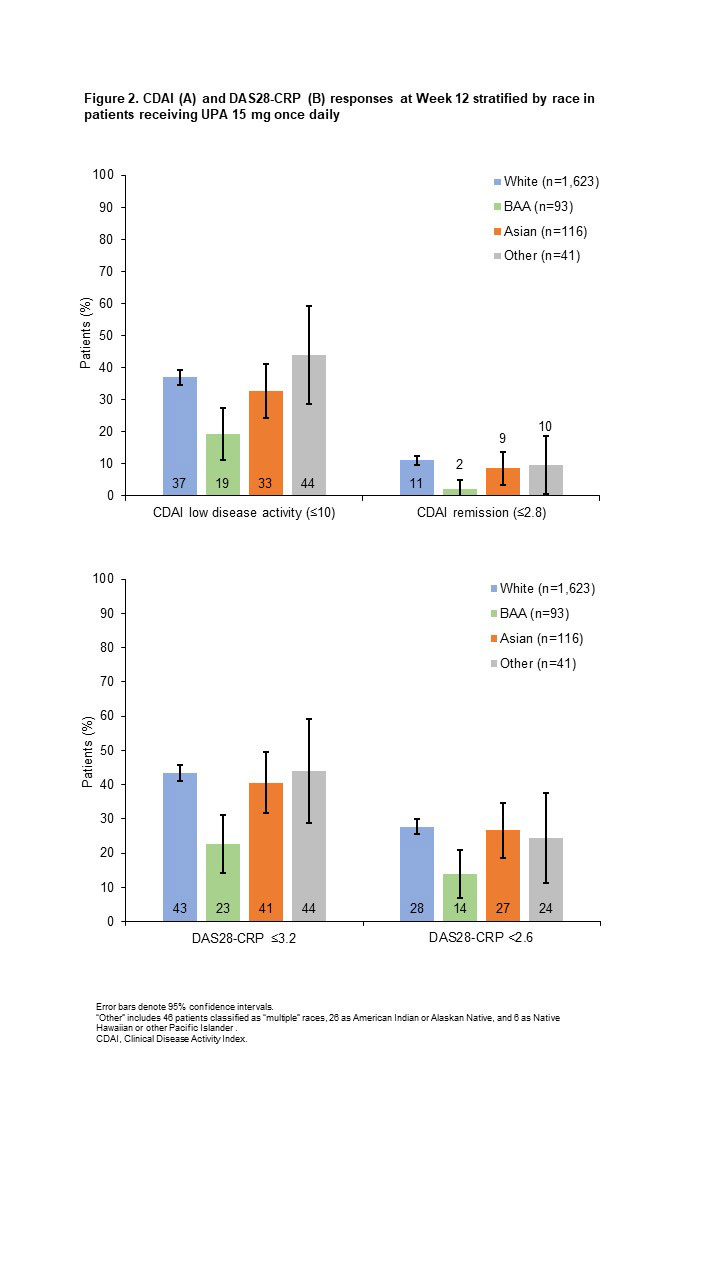
Results: Efficacy was analyzed in 1,873 patients (White, n=1,623; BAA, n=93; Asian, n=116; Other, n=41). Baseline characteristics were generally similar across groups, although White and BAA patients had a longer disease duration, and higher proportions of BAA and Other patients had a BMI ≥30 kg/m2. ACR20/50/70 responses (20%/50%/70% improvement in ACR scores) with UPA were generally similar for White, Asian, and Other patients, but lower for BAA patients, particularly ACR70 (Figure 1). Similar patterns were observed for achieving Clinical Disease Activity Index low disease activity and remission (Figure 2A) and 28-joint DAS with CRP (DAS28-CRP) ≤3.2 and < 2.6 (Figure 2B). Least squares mean change from baseline at Week 12 in the HAQ-Disability Index score was greatest for Other patients, followed by Asian, White, and BAA patients (−0.86 [n=35], −0.77 [n=86], −0.67 [n=1,372], and −0.33 [n=74], respectively). Reductions in patient’s global assessment of pain were similar for Asian, Other, and White groups (−35.82 [n=86], −35.27 [n=35], and −33.42 [n=1,374], respectively), with the lowest reduction observed in BAA patients (−23.13 [n=74]). Safety was assessed in 4,097 patients (White, n=3,573; BAA, n=223; Asian, n=227; Other, n=74). Overall AE rates were higher among non-White vs White patients, whereas rates of serious AEs and AEs leading to treatment discontinuation were generally similar across groups (Table 1). Most AEs of special interest occurred infrequently, although rates of serious infection and herpes zoster were highest in Asian patients, and rates of creatine phosphokinase (CPK) elevations and adjudicated venous thromboembolic events were highest in BAA patients.
Conclusion: In patients with RA, UPA was efficacious across all race groups; however, efficacy was generally lowest in BAA patients vs other race groups. Safety was comparable across groups, except for a higher overall AE rate among non-White patients and higher rates of some AEs of special interest in Asian and BAA patients. Results should be interpreted with respect to small patient numbers; future studies should evaluate factors underlying racial disparities in larger populations.
References1. Wright G, et al. Arthritis Rheumatol 2021;73(suppl 10). Abstract 1680.
To cite this abstract in AMA style:
Wright G, Mysler E, Navarro-Millan I, Tanaka Y, Anyanwu S, Liu J, Tanjinatus O, Garrison A, Wells A. Efficacy and Safety of Upadacitinib in Patients Across Races with Rheumatoid Arthritis: A Post Hoc Analysis of Six Phase 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-across-races-with-rheumatoid-arthritis-a-post-hoc-analysis-of-six-phase-3-clinical-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-across-races-with-rheumatoid-arthritis-a-post-hoc-analysis-of-six-phase-3-clinical-trials/