Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The tofacitinib modified-release (MR) 11 mg once daily (QD) formulation was first approved in the US in 2016 for the treatment of patients (pts) with moderate to severe RA and an inadequate response or intolerance to MTX. ORAL Shift is the first global study of tofacitinib MR 11 mg QD + MTX. Efficacy and pt-reported outcomes (PROs), and safety, from the open-label (OL) phase of ORAL Shift are reported here.
Methods: ORAL Shift (NCT02831855) was a global Phase 3b/4 study in pts aged ≥ 18 years with moderate to severe RA and an inadequate response to MTX. Pts received OL tofacitinib MR 11 mg QD + MTX (tofacitinib + MTX) for 24 weeks; those achieving low disease activity (LDA; Clinical Disease Activity Index [CDAI] ≤ 10) at Week (W)24 entered the 24-week double-blind (DB) MTX withdrawal phase, where the primary endpoint was assessed (data reported elsewhere).1 Outcomes from the OL phase, reported descriptively here, include: mean change from baseline (BL) to W12 and W24 in disease activity measures, and PROs; rates of ACR and HAQ-Disability Index (DI) response, and LDA and remission, at W12 and W24 (see Table 1); and safety (see Table 2).
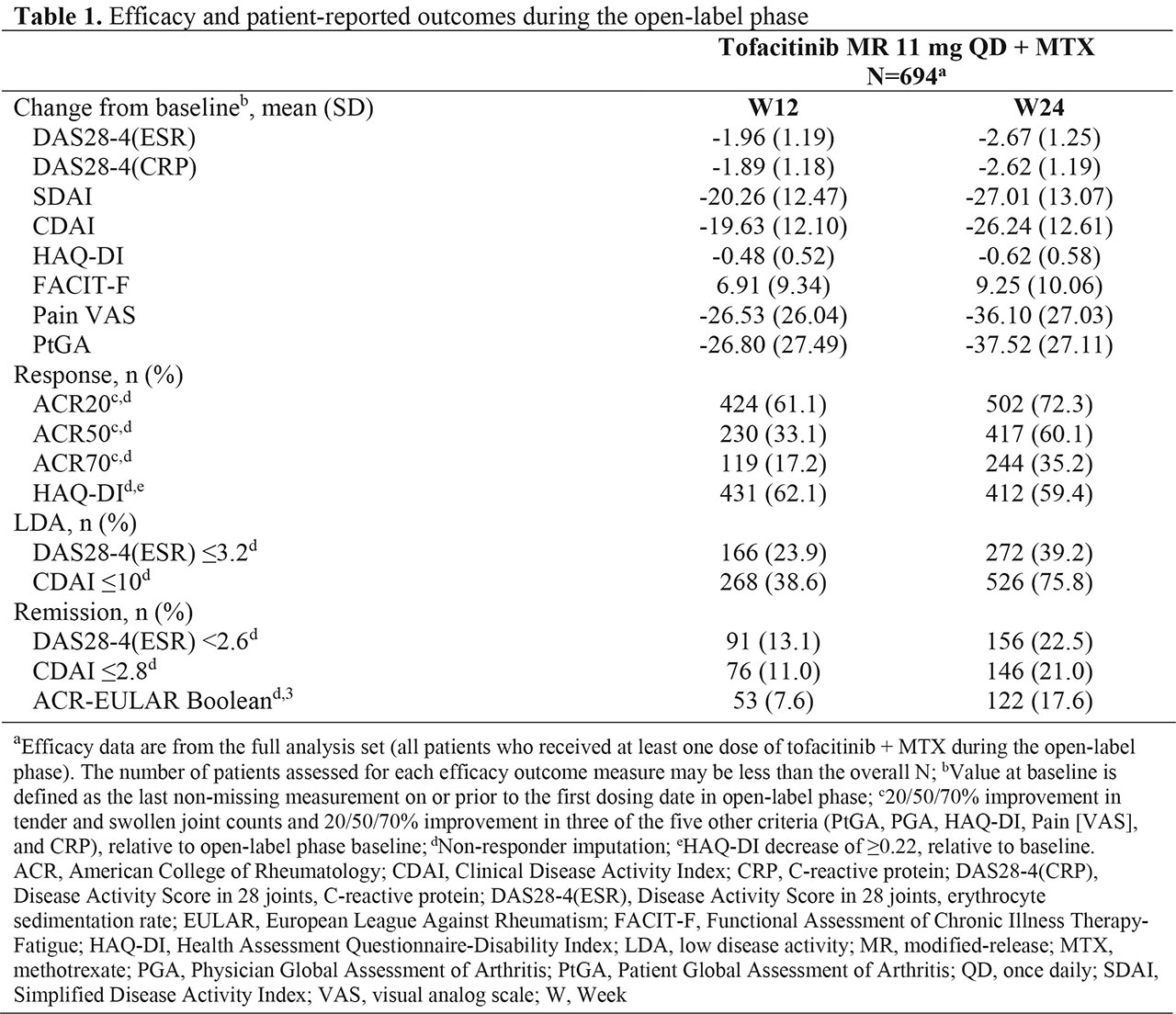
Results: In the OL phase, 694 pts received tofacitinib + MTX. Most pts were female (76.7%), white (85.6%), with a mean age of 56.8 years and mean RA duration of 8.8 years. At W24 (end of the OL phase), 526 (75.8%) pts achieved CDAI-defined LDA (Table 1). In the OL phase, Disease Activity Score in 28 joints, ESR (DAS28-4[ESR]) improved with mean (standard deviation) changes from BL of ‑1.96 (1.19) and -2.67 (1.25) at W12 and W24, respectively (Table 1). Mean changes from BL at W12 and W24 showed improvements in all other efficacy outcomes and PROs (Table 1). ACR20/50/70 and HAQ-DI response rates, and LDA and remission rates, improved from W12 to W24 (Table 1). Adverse events (AEs), serious AEs, and discontinuations due to AEs were reported by 52.2%, 2.9%, and 5.9% of pts, respectively; no deaths were reported (Table 2). The most common AEs were nasopharyngitis and upper respiratory tract infections. AEs of special interest each occurred in ≤ 1% of pts (Table 2).
Conclusion: Tofacitinib MR 11 mg QD + MTX improved disease activity and PROs in pts with moderate to severe RA and an inadequate response to MTX. No new safety risks were observed. Efficacy and safety in this OL phase appeared consistent with that of tofacitinib immediate-release 5 mg twice daily2 and with the DB phase of ORAL Shift.1
- Cohen SB et al. Ann Rheum Dis 2019; 78: A260.
- Bird P et al. J Clin Rheumatol 2019; 25: 115-126.
- Felson DT et al. Arthritis Rheum 2011; 63: 573-586.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Jennifer Arnold of CMC Connect and funded by Pfizer Inc.
To cite this abstract in AMA style:
Cohen S, Pope J, Haraoui B, Mysler E, Diehl A, Lukic T, Liu S, Stockert L, Menon S, Keystone E. Efficacy and Safety of Tofacitinib Modified-Release 11 Mg Once Daily + MTX in RA Patients with an Inadequate Response to MTX: Open-Label Phase Results from a Global Phase 3b/4 MTX Withdrawal Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-modified-release-11-mg-once-daily-mtx-in-ra-patients-with-an-inadequate-response-to-mtx-open-label-phase-results-from-a-global-phase-3b-4-mtx-withdrawal-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-modified-release-11-mg-once-daily-mtx-in-ra-patients-with-an-inadequate-response-to-mtx-open-label-phase-results-from-a-global-phase-3b-4-mtx-withdrawal-study/