Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: In patients (pts) with PsA and AS, smoking may be associated with an increased comorbidity burden, and greater responses to TNF inhibitors (TNFi) are observed in never vs past/current smokers in real-world studies. This post hoc analysis assessed the efficacy and safety of tofacitinib in randomized controlled trials (RCTs) of pts with PsA or AS by history of cigarette smoking.
Methods: Pooled data from 3 Phase (P)3 trials of pts with PsA and 1 P2/1 P3 trial of pts with AS, receiving tofacitinib 5 or 10 mg (PsA only) twice daily (BID) or placebo (PBO), were assessed by baseline (BL) smoking status (ever [past/current] vs never smoker). Efficacy outcomes (assessed by simple means): ACR50 and PASDAS ≤ 3.2 responses, and change from BL (Δ) in HAQ-DI to Month (M)6 in pts with PsA; and ASAS40 and BASDAI50 responses, and ΔBASFI to M4 (to M3 only in P2 trial) in pts with AS. Exploratory efficacy analysis by current/past/never smoker BL status was performed. Safety was assessed to M3 (PsA pts)/M4 (AS pts). Data as observed; no imputation for missing data.
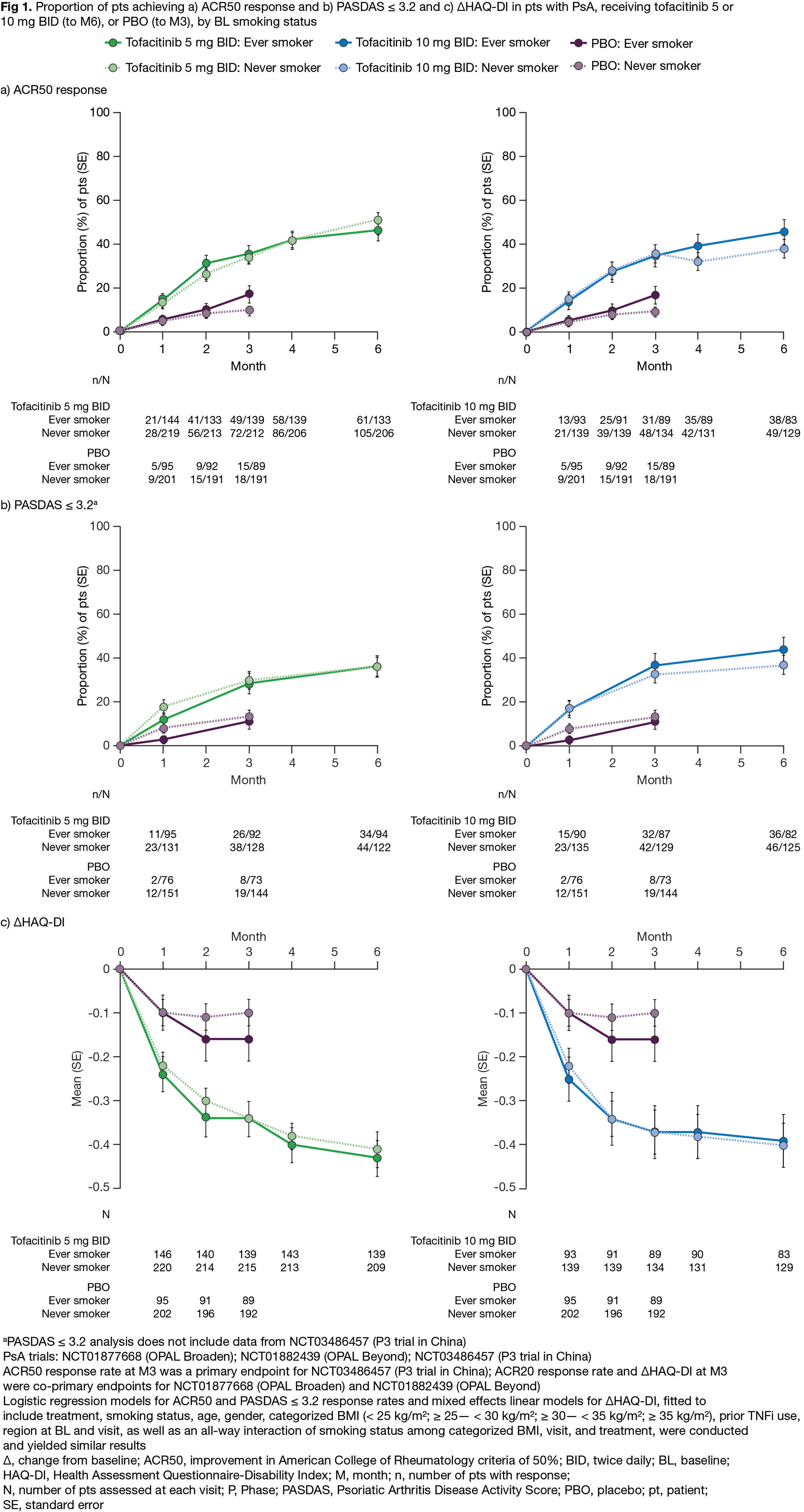
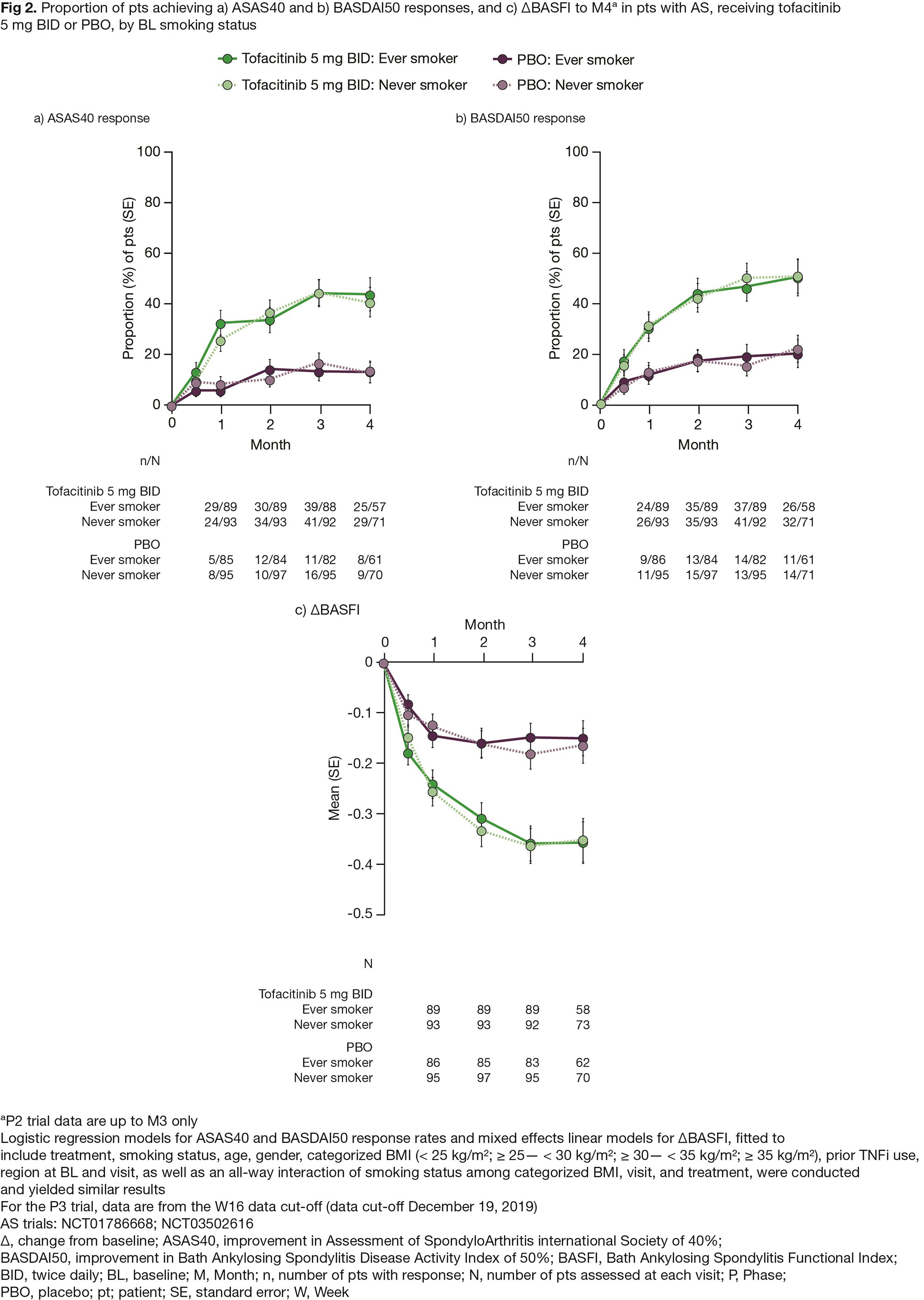
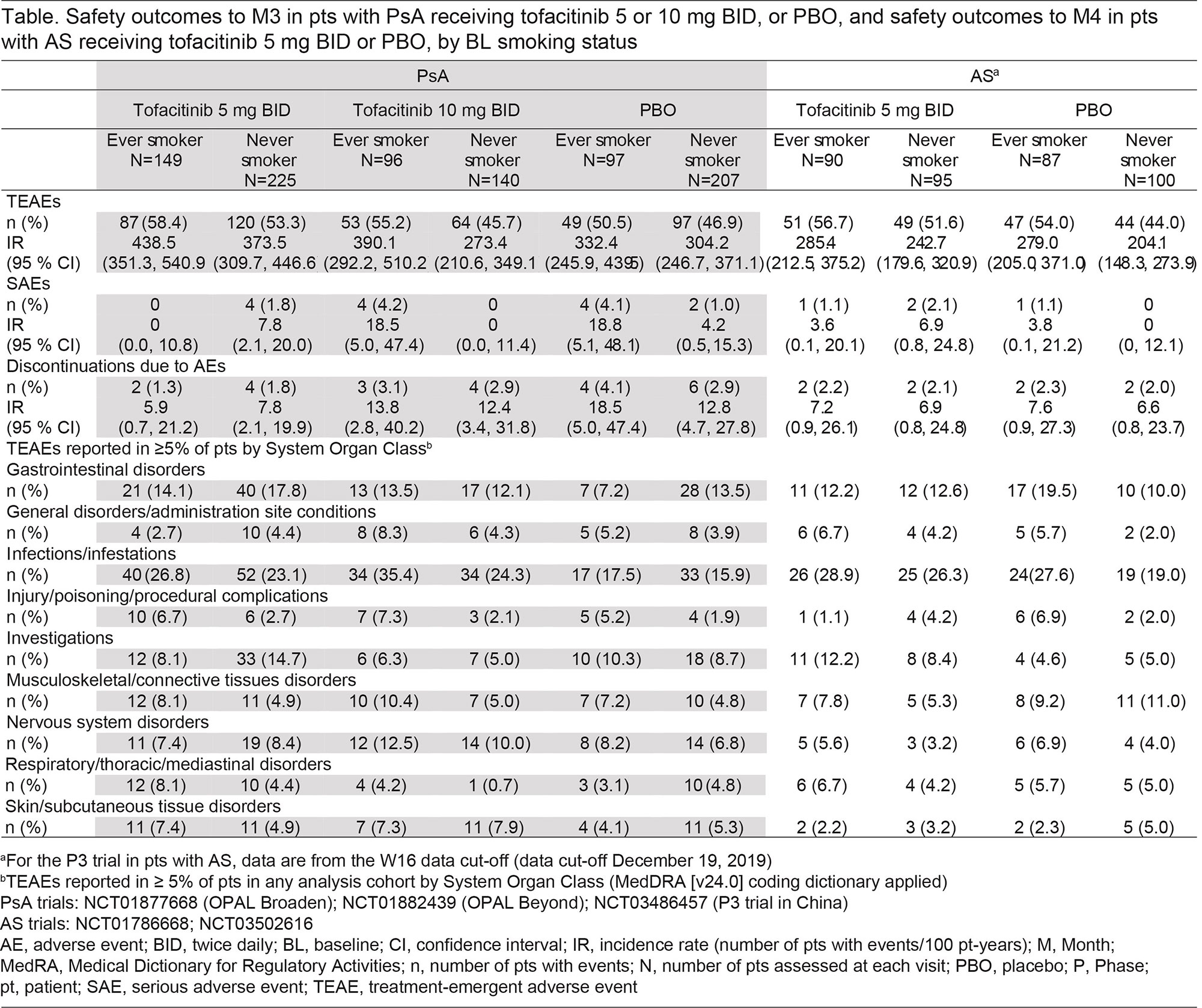
Results: PsA and AS cohorts included 914 (342 ever/572 never smokers) and 372 (177 ever/195 never smokers) pts, respectively. Overall, BL characteristics were generally similar between ever vs never smokers, except for a higher proportion of females in never vs ever smoker categories (PsA, 57.7% vs 42.7%; AS, 25.1% vs 15.8%), and a longer disease duration in ever vs never smoker categories (mean [SD]: PsA, 7.6 [7.0] vs 7.0 [7.2] years; AS, 8.0 [8.2] vs 6.8 [7.6] years). In both smoking categories, efficacy was greater for tofacitinib (5 and 10 mg [PsA only] BID) vs PBO (Figs 1 and 2). Across treatments, efficacy was generally comparable in ever vs never smokers, when considering ACR50 and PASDAS ≤ 3.2 response rates, and ΔHAQ-DI to M6 in pts with PsA (Fig 1), as well as ASAS40 and BASDAI50 response rates, and ΔBASFI to M4 in pts with AS (Fig 2). Exploratory analyses showed that, across treatments, efficacy was comparable in current/past/never BL smokers (data not shown). To M3/M4 in pts with PsA/AS, incidence rates (IRs) of treatment-emergent adverse events (TEAEs) were numerically higher in ever vs never smokers (all treatments); and there were no consistent trends across treatments between ever vs never smokers for IRs of serious AEs and discontinuations due to AEs, though numbers of events were small (Table). Generally, higher proportions of infections/infestations, injury/poisoning/procedural complications, musculoskeletal/connective tissue disorders, and respiratory/thoracic/mediastinal disorders were observed in ever vs never smokers (Table). Sample sizes were small for some groups, following categorization by BL smoking status; results should be interpreted cautiously.
Conclusion: In this post hoc analysis of RCTs of pts with PsA and AS, tofacitinib efficacy was comparable in ever vs never smokers, which contrasts with reports from real-world studies of TNFi-treated pts. TEAEs were numerically higher in ever vs never smokers, complementing prior reports of an association between smoking and comorbidities in pts with PsA and AS.Study sponsored by Pfizer. Medical writing support provided by J Juana, CMC Connect, and K Munn, on behalf of CMC Connect, and funded by Pfizer.
To cite this abstract in AMA style:
Ogdie A, Kristensen L, Soriano E, Akar S, Sun Y, Gruben D, Fallon L, Kinch C, Gladman D. Efficacy and Safety of Tofacitinib in Patients with Psoriatic Arthritis or Ankylosing Spondylitis by History of Cigarette Smoking [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-psoriatic-arthritis-or-ankylosing-spondylitis-by-history-of-cigarette-smoking/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-psoriatic-arthritis-or-ankylosing-spondylitis-by-history-of-cigarette-smoking/