Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Miscellaneous Rheumatic and Inflammatory Diseases II
Session Type: Abstract Session
Session Time: 10:30AM-12:00PM
Background/Purpose: Polymyalgia Rheumatica (PMR) is a common inflammatory disease in elderly persons whose pathogenesis is unclear. Glucocorticoids are the first-line drugs for patients with PMR, but resulting in numerous side effects. As JAK kinase contributes to the inflammatory response, we explore whether JAK-inhibitor tofacitinib can treat patients with PMR.
We aimed to explore the pathogenetic features of PMR. We assessed the efficacy and safety of tofacitinib in patients with PMR (ChiCTR2000038253).
Methods: The gene expression profiles were analyzed in peripheral blood mononuclear cells (PBMCs) in patients with newly diagnosed PMR and sex-matched healthy controls (HC) by RNA sequencing and were confirmed by RT-PCR.
Patients with newly diagnosed PMR were randomized to tofacitinib (5mg bid) group and Glucocorticoids (Pred 15mg/day, gradually tapered) group. All PMR patients underwent clinical and laboratory examinations at 0, 4, 8, 12, 16, 20, and 24 weeks, and PMR activity disease scores (PMR-AS) were also calculated. The primary endpoint was the proportion of patients with PMR-AS≤10 at weeks 12 and 24. Secondary endpoints: PMR-AS score, CRP, and ESR at weeks 12 and 24.
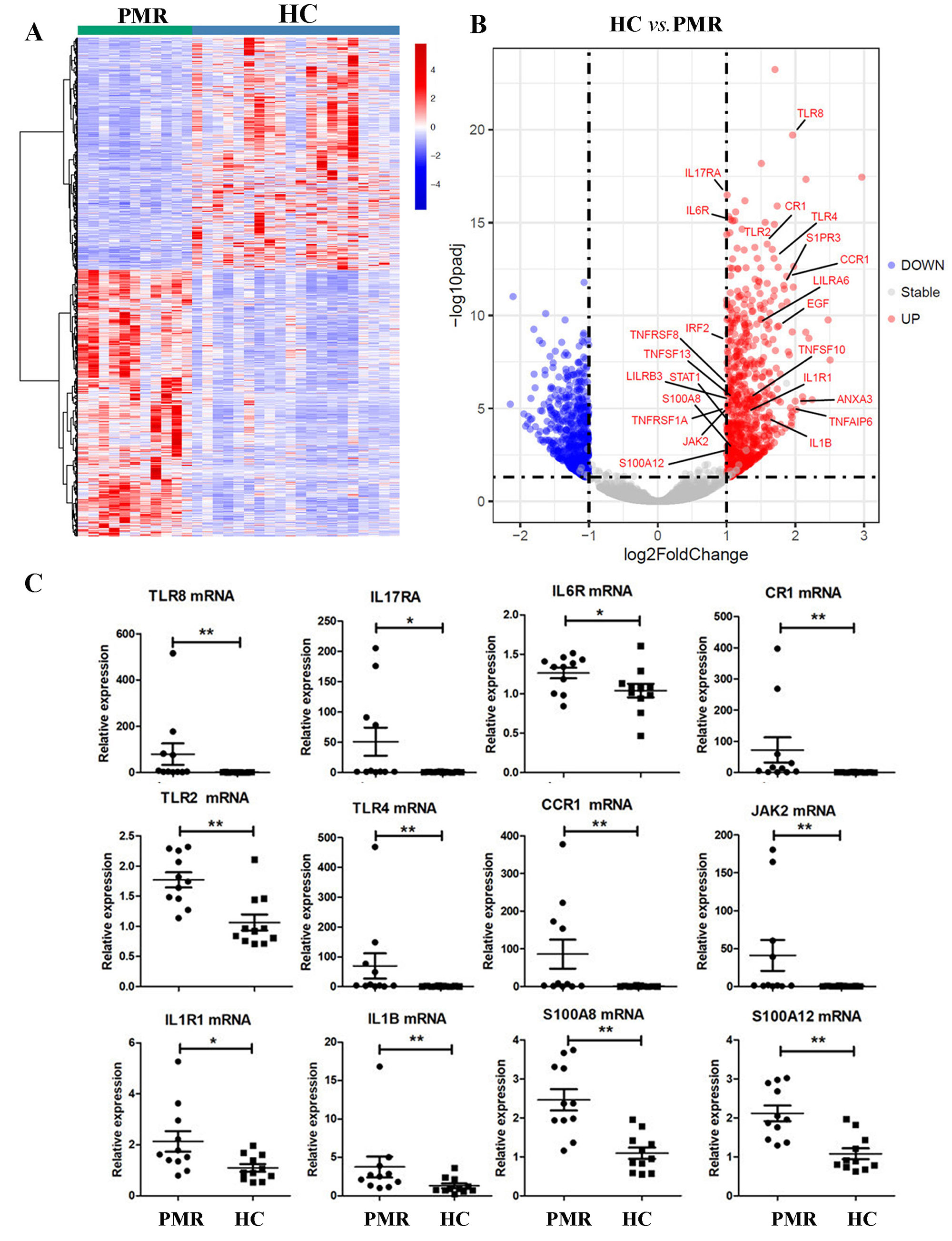
Results: The gene expressions of PBMCs in 11 patients with newly diagnosed PMR were significantly different from those in 20 HC by RNA sequencing (Figure 1A). GO and KEGG analysis demonstrated that inflammatory response and cytokine-cytokine receptor interaction were the most remarkable pathways (data not shown). There were markedly expanded IL6R, IL1B, IL1R1, JAK2, TLR2, TLR4, TLR8, CCR1, CR1, S100A8, S100A12, and IL17RA expressions (Figure 1B, C). Those genes may trigger the JAK signaling.
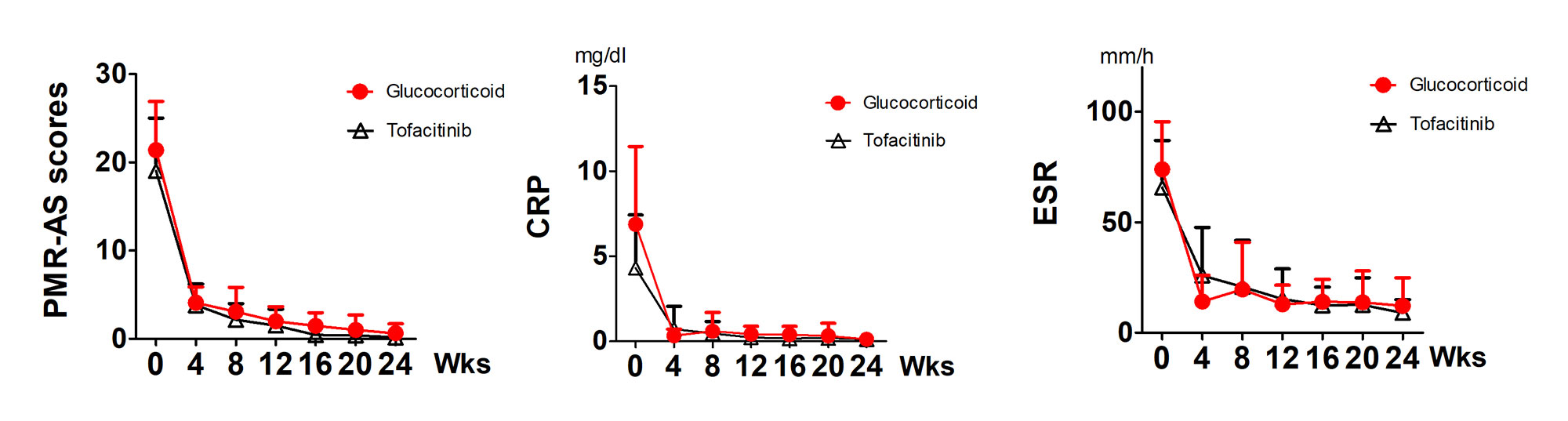
27 patients with newly diagnosed PMR received the tofacitinib and 25 patients received glucocorticoid. No significant difference in gender ratio and age was found between the two groups. The baseline of PMR-AS was comparable in tofacitinib (19.02±5.98) and glucocorticoid group (21.38±5.52). At weeks 12 and 24, all patients in both groups had PMR-AS< 10. PMR-AS, CRP, and ESR were all significantly decreased at weeks 12, and 24 in both groups (Figure 2).
Only one patient in tofacitinib group did not respond well after 12 weeks, and one patient in glucocorticoid group did not respond well after 16 weeks. One patient had a mild urinary infection after 4 weeks treatment of tofacitinib, and recovered quickly to antibiotic without treatment interruption of tofacitinib. One patient had an increased blood pressure after 2 weeks treatment of glucocorticoid, falling back to normal level after additional antihypertensive therapy.
Conclusion: Many inflammatory genes associated with JAK signaling were increased in patients with PMR, suggesting an important role of JAK signaling in the development of PMR disease. We found that tofacitinib effectively treats patients with PMR as glucocorticoid does. No severe side effects were found in 24 weeks period treatment of tofacitinib.
To cite this abstract in AMA style:
Ma X, Yang F, Lin J, Chen W. Efficacy and Safety of Tofacitinib in Patients with Polymyalgia Rheumatica (EAST PMR): A Prospective Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-polymyalgia-rheumatica-east-pmr-a-prospective-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-patients-with-polymyalgia-rheumatica-east-pmr-a-prospective-study/