Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Juvenile idiopathic arthritis (JIA) encompasses various chronic inflammatory conditions in children that lead to joint discomfort and potential disability. Tocilizumab, an antibody targeting interleukin-6, has shown effectiveness in treating rheumatoid arthritis and may also help children with JIA. Due to the distinct characteristics of the disease, its efficacy and safety in children require assessment.
Methods: A systematic literature review was conducted by searching PUBMED, EMBASE, COCHRANE, SCIENCE DIRECT, and SCOPUS for RCTs and non-RCTs assessing the efficacy and safety of Tocilizumab in Juvenile Idiopathic Arthritis up to 2025. The primary outcomes assessed were disease activity improvement, joint inflammation reduction, Functional enhancements (i.e., CHAQ score), and QOL scores. Statistical analysis was conducted in R (v2025.05.0+496).
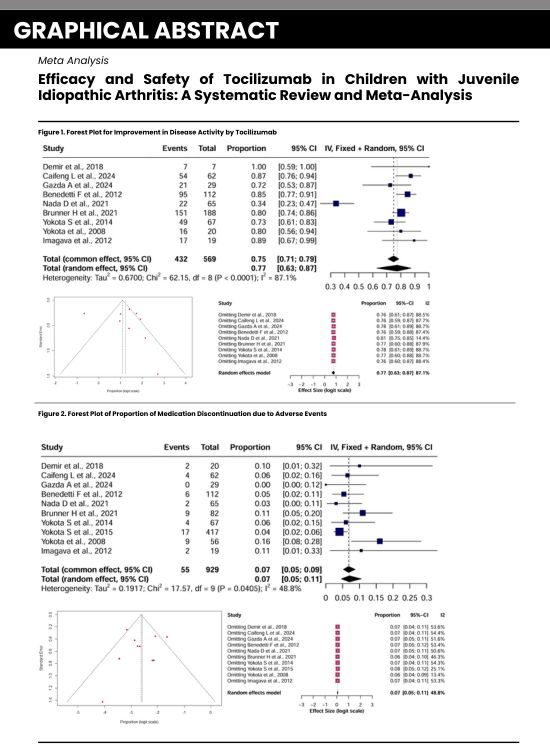
Results: A meta-analysis involving 11 studies with a total of 929 children diagnosed with Juvenile Idiopathic Arthritis (JIA) indicated that Tocilizumab led to improved disease activity, achieving a pooled risk ratio of 3.77 (95% CI 2.32-6.13, p=0.060) when compared to the control group. When examining study types, the subgroup analysis revealed proportions of 0.04 (95% CI 0.03-0.07) for non-RCTs and 0.10 (95% CI 0.07-0.14) for RCTs (p=0.0040). The average Childhood Health Assessment Questionnaire (CHAQ) score was 1.67, indicating functional improvement; the quality of life score was 45.74. In terms of JIA subtypes, the proportions were 0.11 (95% CI 0.06-0.18) for polyarticular JIA and 0.06 (95% CI 0.04-0.08) for systemic JIA (p=0.0502). Adverse events were reported in 7% of the participants (pooled proportion 0.07, 95% CI 0.05-0.09), primarily mild infections, with no serious adverse events noted. Sensitivity analysis demonstrated the stability of the results (range 0.06-0.07), with moderate heterogeneity (I²=48.8%).
Conclusion: Our findings support using Tocilizumab in managing Juvenile Idiopathic Arthritis, demonstrating improved outcomes without significantly increasing adverse events. These results offer a clearer understanding of its clinical value and underscore the need for further high-quality randomized trials.
To cite this abstract in AMA style:
Jahangir K, Murtaza M, Jannat R, Arham M, Gohar N, Haider F, Ahmed R, Raja F, Habib R, Umar M, Saleem H, Ahmed F, Mirza T, Siddiqui M, Umer M, Ahmed H, Anwar M, Bint e Tahir R, Junaid Y, Ali R. Efficacy and Safety of Tocilizumab in Children with Juvenile Idiopathic Arthritis: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-tocilizumab-in-children-with-juvenile-idiopathic-arthritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tocilizumab-in-children-with-juvenile-idiopathic-arthritis-a-systematic-review-and-meta-analysis/