Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: To evaluate the efficacy and safety of telitacicept in adult patients with primary Sjögren’s syndrome (pSS) in a phase II randomized double-blind placebo-controlled trial.
Methods: Patients with pSS with positive anti-SSA antibody and ESSDAI≥5 were randomly assigned, in a 1:1:1 ratio, to receive weekly subcutaneous telitacicept 240 mg, 160 mg, or placebo for 24 weeks. The primary endpoint was the change from baseline in the ESSDAI at week 24. Safety was monitored.
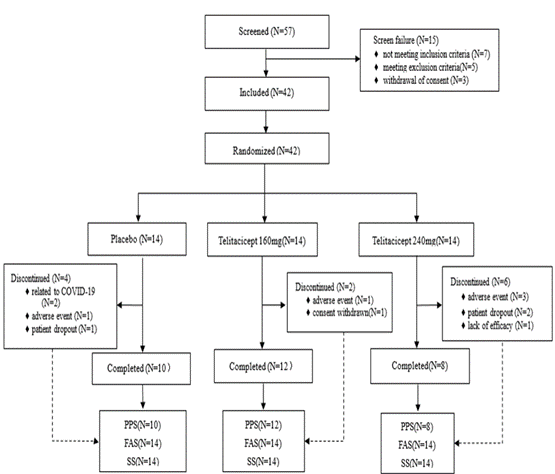
Results: 42 patients were enrolled and randomized (n=14 per group). Thirty patients who completed their 24 weeks visit were included in the per-protocol set (PPS) (telitacicept 240 mg, n=8; telitacicept, 160 mg; n=12; placebo, n=10).
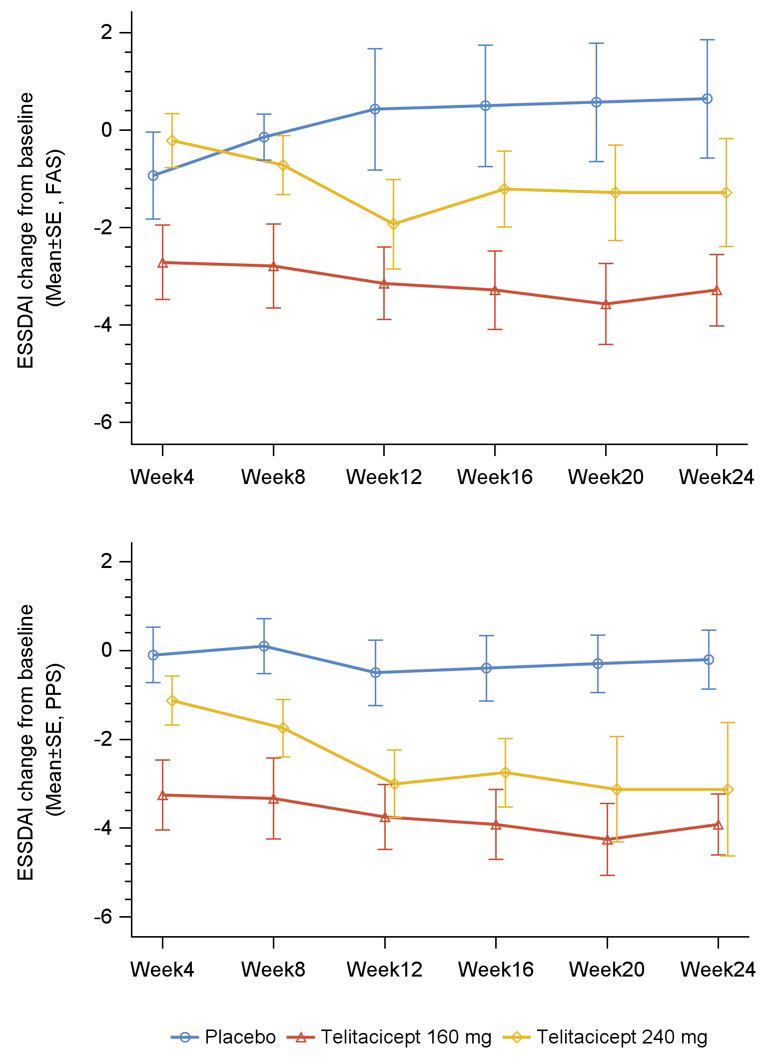
Administration of telitacicept 160 mg resulted in a significant reduction in ESSDAI score from baseline to week 12 and week 24 compared with placebo in FAS and PPS populations (p< 0.05); telitacicept 240 mg resulted in a significant reduction in ESSDAI score from baseline to week 24 compared with placebo in the PPS population and week 12 in the FAS population (p< 0.05).
Significantly greater reductions from baseline in MFI-20 to weeks 12 and 24 were observed in patients in the telitacicept group (p< 0.05).
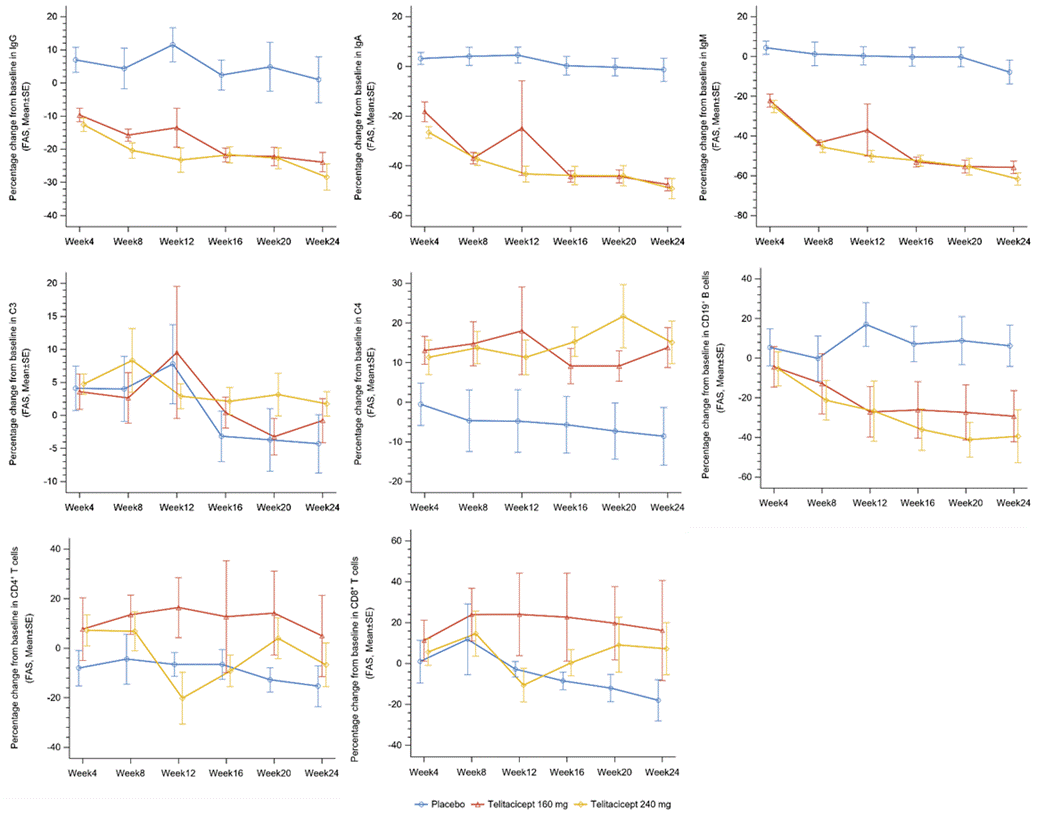
Telitacicept induced significant reductions in serum Ig levels at each visit throughout the 24 weeks. No serious adverse events were observed in the telitacicept treating group.
Conclusion: Telitacicept showed clinical benefits and good safety in the treatment of pSS.
To cite this abstract in AMA style:
Xu D, Zhang S, Huang C, Huang C, Qin L, Li X, Chen M, Liu X, Liu Y, Li Z, Hu J, Bao C, Wei w, Tian J, Duan X, Fang J, Zeng X. Efficacy and Safety of Telitacicept in Primary Sjögren’s Syndrome: A Randomized, Double-blind, Placebo-controlled, Phase 2 Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-telitacicept-in-primary-sjogrens-syndrome-a-randomized-double-blind-placebo-controlled-phase-2-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-telitacicept-in-primary-sjogrens-syndrome-a-randomized-double-blind-placebo-controlled-phase-2-trial/